FDA Approved Biologics and Pending Applications
FDA Approved Biologics and Pending Applications
erenumab-aooe
BL 761077
adalimumab-atto
aBL 761024
darbepoetin alfa
BL 103951
infliximab-axxq
aBL 761086
eculizumab-aeeb
aBL 761333
blinatumomab
BL 125557
etanercept
BL 103795
epoetin alfa
BL 103234
romosozumab-aqqg
BL 761062
tarlatamab-dlle
BL 761344
talimogene laherparepvec
BL 125518
trastuzumab-anns
aBL 761073
bevacizumab-awwb
aBL 761028
pegfilgrastim
BL 125031
filgrastim
BL 103353
romiplostim
BL 125268
aflibercept-ayyh
aBL 761298
denosumab
BL 125320
evolocumab
BL 125522
rituximab-arrx
aBL 761140
panitumumab
BL 125147
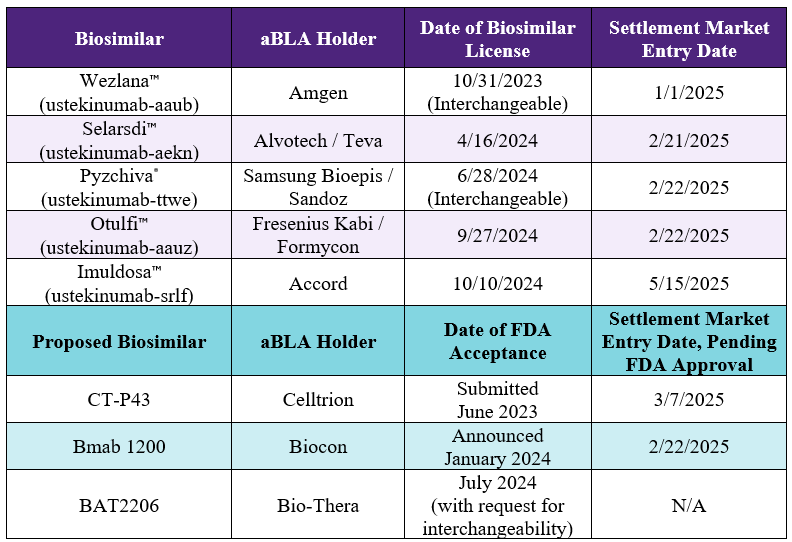
ustekinumab-auub
aBL 761285 / aBL 761331
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Amgevita (adalimumab) (Amgen) (November-2020)Avsola® (infliximab) (Amgen) (March-2020)Kanjinti® (trastuzumab) (Amgen) (February-2020)Mvasi® (bevacizumab) (Amgen) (October-2018)Pavblu (aflibercept) (Amgen) (July-2025)Wezlana (ustekinumab) (Amgen) (December-2023)
Biosimilars Approved In The E.U.
Amgevita / Solymbic (adalimumab) (Amgen) (March-2017)Bekemv (eculizumab) (Amgen) (April-2023) Kanjinti® (trastuzumab) (Amgen / Allergan) (May-2018)Mvasi® (bevacizumab) (Amgen/Allergan) (January-2018)Pavblu (aflibercept) (Amgen) (April-2025)Wezenla (ustekinumab) (Amgen) (June-2024)
Biosimilars Approved In Australia
Amgevita (adalimumab) (Amgen) (November-2017) Kanjinti® (trastuzumab) (Amgen) (May-2019)Mvasi® (bevacizumab) (Amgen) (November-2019)Wezlana (ustekinumab) (Amgen) (January-2024)
 Inter Partes Review Proceedings
Inter Partes Review Proceedings
ENBRELIPR2015-01792IPR2017-01916IPR2017-02066OPDIVOIPR2025-00601IPR2025-00602IPR2025-00603HUMIRA / AMJEVITAIPR2015-01514IPR2015-01517NEULASTA / STIMUFENDIPR2019-00971IPR2019-01183IPR2020-00314NEUPOGEN / NEULASTA / GRASTOFIL / LAPELGAIPR2016-01542NEUPOGEN / NEULASTA / NIVESTYM / NYVEPRIAIPR2021-00528NEUPOGEN / NEULASTA / PEGFILGRATIM (LUPIN)IPR2021-00326NEUPOGEN / NEULASTA / RELEUKO / FYLNETRAIPR2019-00791IPR2019-00797NEUPOGEN / NEULASTA / RELEUKO / GRASTOFIL / LAPELGA / FYLNETRAPGR2019-00001NEUPOGEN / NEULASTA / STIMUFENDIPR2019-01183SOLIRIS / BKEMVIPR2019-00739IPR2019-00740IPR2019-00741
 U.S. Patent Litigations
U.S. Patent Litigations
BLINCYTO1:22-cv-00035 (D. Del.)DUPIXENT1:17-cv-10465 (D. Mass.)2:17-cv-02613 (C.D. Cal.)ENBREL1:09-cv-00805 (D. Del.)2:16-cv-01118 (D.N.J.) 2:19-cv-11755 (D.N.J.) 3:13-cv-02904 (N.D. Cal.) EVENITY1:23-cv-10861 (D. Mass.)NEUPOGEN0:15-cv-62081 (S.D. Fla.)0:18-cv-61828 (S.D. Fla.)1:10-cv-01540 (E.D. Ill.)1:18-cv-01064 (D. Del.)1:20-cv-00561 (D. Del.)2:13-cv-04911 (D.N.J.)2:18-cv-03347 (D.N.J.)3:14-cv-04741 (N.D. Cal.) 3:19-cv-00977 (N.D. Cal.) 3:19-cv-01374 (S.D. Cal.)REPATHA1:14-cv-01317 (D. Del.)1:14-cv-01349 (D. Del.)1:14-cv-01393 (D. Del.)1:14-cv-01414 (D. Del.)2:21-cv-01816 (C.D. Cal.)AMJEVITA / YUSIMRY1:19-cv-00139 (D. Del.)AVASTIN / MVASI1:17-cv-00165 (D. Del.)1:17-cv-01407 (D. Del.)1:17-cv-01471 (D. Del.)1:19-cv-00602 (D. Del.)2:17-cv-07349 (C.D. Cal.)AVASTIN / MVASI / GENENTECH CABILLY1:17-cv-01407 (D. Del.)1:17-cv-01471 (D. Del.)1:19-cv-00602 (D. Del.)2:17-cv-07349 (C.D. Cal.)ENBREL / ERELZI2:16-cv-01118 (D.N.J.) 3:13-cv-02904 (N.D. Cal.) ENBREL / ETICOVO2:19-cv-11755 (D.N.J.) EPOGEN / PROCRIT / RETACRIT1:15-cv-00839 (D. Del.)EYLEA / PAVBLU1:24-cv-00039 (N.D.W. Va.) (transferred from 1:24-cv-00264 (C.D. Cal.)) / MDL 1:24-md-03103 (N.D.W. Va.)1:25-cv-00074 (N.D.W. Va.) (transferred from 2:25-cv-05499 (C.D. Cal.)) / MDL 1:24-md-03103 (N.D.W. Va.)GRANIX / NEUPOGEN / NEULASTA2:13-cv-04911 (D.N.J.)HERCEPTIN / KANJINTI / GENENTECH CABILLY1:18-cv-00924 (D. Del.)HUMIRA / AMJEVITA1:16-cv-00666 (D. Del.)NEULASTA / FULPHILA2:17-cv-01235 (W.D. Pa.)NEULASTA / LAPELGA0:15-cv-61631 (S.D. Fla.) NEULASTA / NYVEPRIA1:20-cv-00201 (D. Del.)NEULASTA / UDENYCA1:17-cv-00546 (D. Del.)56-2017-00493553-CU-BT-VTA (CA Sup. Ct.)NEULASTA / ZIEXTENZO2:16-cv-01276 (D.N.J.)3:16-cv-02581 (N.D. Cal.)3:19-cv-00977 (N.D. Cal.) NEUPOGEN / GRASTOFIL0:15-cv-62081 (S.D. Fla.)NEUPOGEN / NEULASTA / GRASTOFIL / LAPELGA0:18-cv-61828 (S.D. Fla.)NEUPOGEN / NIVESTYM1:18-cv-01064 (D. Del.)1:20-cv-00561 (D. Del.)NEUPOGEN / NYPOZI3:19-cv-01374 (S.D. Cal.)NEUPOGEN / RELEUKO2:18-cv-03347 (D.N.J.)NEUPOGEN / ZARXIO3:14-cv-04741 (N.D. Cal.) 3:19-cv-00977 (N.D. Cal.) NEUPOGEN / ZARXIO / NEULASTA / ZIEXTENZO3:19-cv-00977 (N.D. Cal.) PRALUENT / REPATHA1:14-cv-01317 (D. Del.)1:14-cv-01349 (D. Del.)1:14-cv-01393 (D. Del.)1:14-cv-01414 (D. Del.)PROLIA / XGEVA / AVT031:25-cv-17277 (D.N.J.) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / BILDYOS / BILPREVDA1:25-cv-12160 (D.N.J.) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / BONCRESA / OZILTUS1:25-cv-17278 (D.N.J.) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / BOSAYA / AUKELSO1:25-cv-13358 (D.N.J.) (transferred from 1:25-cv-11867 (D. Mass.) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / CONEXXENCE / BOMYNTRA1:25-cv-01080 (D.N.J.) (transferred from 1:24-cv-09555 (N.D. Ill.)) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / ENOBY / XTRENBO1:25-cv-12152 (D.N.J.) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / ENZ2151:25-cv-17596 (D.N.J.) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / JUBBONTI / WYOST1:23-cv-02406 (D.N.J.)PROLIA / XGEVA / OSPOMYV / XBRYK1:24-cv-08417 (D.N.J.) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / OSVYRTI / JUBEREQ1:25-cv-01305 (D.N.J.) (transferred from 5:24-cv-00642 (E.D.N.C.)) / MDL 1:25-md-03138 (D.N.J.)PROLIA / XGEVA / STOBOCLO / OSENVELT1:24-cv-06497 (D.N.J.) / MDL 1:25-md-03138 (D.N.J.)STELARA / WEZLANA1:22-cv-01549 (D. Del.)