FDA Approved Biologics and Pending Applications
FDA Approved Biologics and Pending Applications
fremanezumab-vfrm
BL 761089
golimumab
AVT05 Approval Pending
aflibercept
AVT06 Approval Pending
reslizumab
BL 761033
eculizumab-aagh
aBL 761340
tbo-filgrastim
BL 125294
trastuzumab-pkrb
aBL 761091
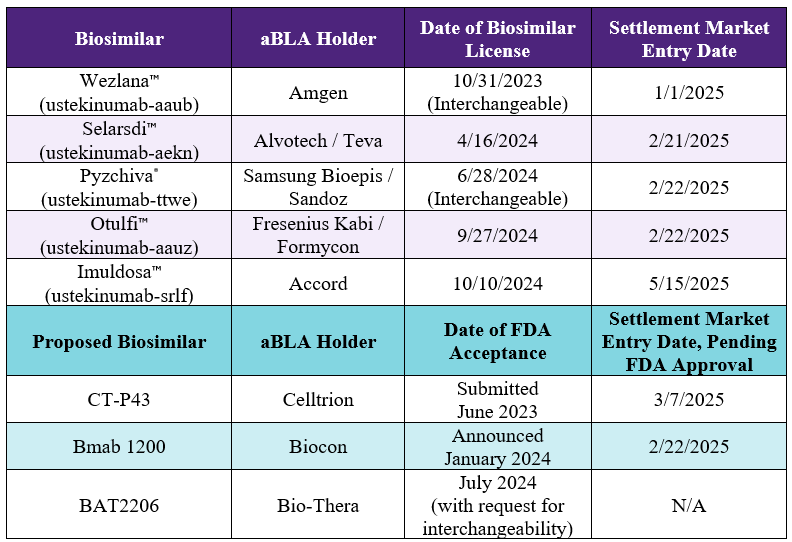
ustekinumab-aekn
aBL 761343
adalimumab-ryvk
aBL 761299
teriparatide
ANDA 208569
rituximab-abbs
aBL 761088
denosumab
TVB-009P Approval Pending
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Herzuma® (trastuzumab) (Celltrion / Teva) (September-2019)Ranopto® (ranibizumab) (Bioeq / Teva) (December-2023)Teva-teriparatide (teriparatide) (Teva) (June-2020)
Biosimilars Approved In The E.U.
Ongavia (ranibizumab) (Teva / Bioeq) (May-2022 in the United Kingdom) Ovaleap (follitropin alfa/beta) (Teva Pharma) (September-2013)Ranivisio (ranibizumab) (Teva / Bioeq) (August-2022)Tevagrastim (filgrastim) (Teva Generics) (September-2008)
Biosimilars Approved In Australia
Tevagrastim (filgrastim) (Teva Pharma Australia) (August-2011)
Biosimilars Approved In Japan
Filgrastim BS (filgrastim) (Teva Pharma Japan / Nippon Kayaku) (February-2013)
 Inter Partes Review Proceedings
Inter Partes Review Proceedings
AJOVY / EMGALITYIPR2018-01422IPR2018-01423IPR2018-01424IPR2018-01425IPR2018-01426IPR2018-01427IPR2018-01710IPR2018-01711IPR2018-01712IPR2022-00738IPR2022-00739IPR2022-00796HERCEPTIN / HERZUMAIPR2017-01121IPR2017-01122IPR2017-01139IPR2017-01140IPR2017-01373IPR2017-01374RITUXAN / TRUXIMAIPR2016-01614IPR2016-01667IPR2017-01093IPR2017-01094IPR2017-01095IPR2017-01227IPR2017-01229IPR2017-01230IPR2018-01019
 U.S. Patent Litigations
U.S. Patent Litigations
FORTEO1:16-cv-00596 (S.D. Ind.)AJOVY / EMGALITY1:17-cv-12087 (D. Mass.)1:18-cv-10242 (D. Mass.)1:18-cv-12029 (D. Mass.)1:21-cv-10954 (D. Mass.)GRANIX / NEUPOGEN / NEULASTA2:13-cv-04911 (D.N.J.)HERCEPTIN / HERZUMA / GENENTECH CABILLY1:18-cv-00095 (D. Del.)1:18-cv-01025 (D. Del.)4:18-cv-00274 (N.D. Cal.)RITUXAN / TRUXIMA / GENENTECH CABILLY1:18-cv-00574 (D.N.J.)1:18-cv-11553 (D.N.J.)4:18-cv-00276 (N.D. Cal.)