U.S. License Holder:
AbbVie Inc.
Date of License:
December-31-2002
Last Update:
July-08-2025
 FDA-Approved Indications
FDA-Approved Indications
HUMIRA (adalimumab) is a tumor necrosis factor (TNF) blocker indicated for treatment of:
Rheumatoid Arthritis (RA): Reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active RA;
Juvenile Idiopathic Arthritis (JIA): Reducing signs and symptoms of moderately to severely active polyarticular JIA in patients 2 years of age and older;
Psoriatic Arthritis (PsA): Reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with active PsA;
Ankylosing Spondylitis (AS): Reducing signs and symptoms in adult patients with active AS;
Crohn's Disease (CD): Treatment of moderately to severely active Crohn's disease in adults and pediatric patients 6 years of age and older;
Ulcerative Colitis (UC): Treatment of moderately to severely active ulcerative colitis in adults and pediatric patients 5 years of age and older;
Plaque Psoriasis (Ps): Treatment of adult patients with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy, and when other systemic therapies are medically less appropriate;
Hidradenitis Suppurativa (HS): Treatment of moderate to severe hidradenitis suppurativa in patients 12 years of age and older;
Uveitis (UV): Treatment of non-infectious intermediate, posterior and panuveitis in adults and pediatric patients 2 years of age and older.
 aBLA / 505(b)(2) Activity
aBLA / 505(b)(2) Activity
aBLA / 505(b)(2) Approved by FDA
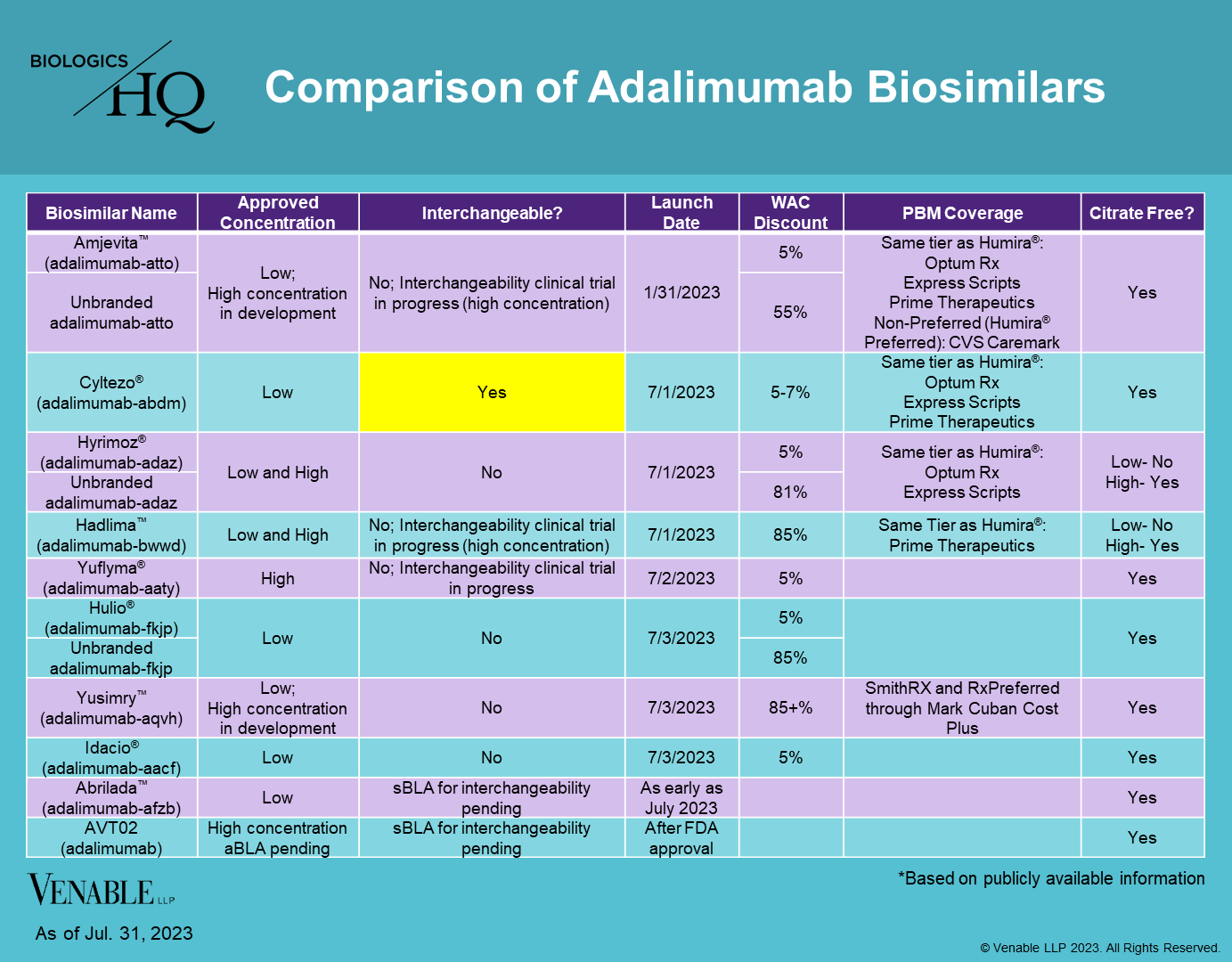
Amjevita: Amgen (September-2016) Cyltezo®: Boehringer Ingelheim (August-2017; Interchangeable October-2021; High-Concentration April-2024) Hyrimoz®: Sandoz (October-2018; High Concentration March-2023; Interchangeable May-2024) Hadlima: Samsung Bioepis / Organon (July-2019, Interchangeable June-2024; High Concentration August-2022, Interchangeable May-2025) Abrilada: Pfizer (November-2019; Interchangeable October-2023) Hulio®: Mylan (July-2020) Yusimry: Coherus (December-2021) Idacio®: Fresenius Kabi (December-2022) Yuflyma®: Celltrion (May-2023, High Concentration Interchangeable) Simlandi®: Alvotech / Teva (February-2024, High Concentration Interchangeable)
aBLA / 505(b)(2) Accepted by FDA
Hadlima: Samsung Bioepis (sBLA for Interchangeability Status: November-2023) Yuflyma®: Celltrion (sBLA for Interchangeability Status: January-2024)
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Adalimumab Injection (Pfizer Canada) (January-2021) Amjevita (Amgen) (November-2020) Hadlima (Samsung Bioepis) (May-2018, high-concentration January-2023) Hulio® (Viatris / Fujifilm Kyowa Kirin) (February-2021) Hyrimoz® (Sandoz Canada) (November-2020) Idacio® (Fresenius Kabi) (October-2020) Simlandi (high-concentration) (Alvotech / JAMP) (January-2022)Yuflyma® (high-concentration) (Celltrion) (December-2021)
Biosimilars Approved In The E.U.
Amgevita / Solymbic (Amgen) (March-2017) Amsparity (Pfizer) (February-2020) Cyltezo (Boehringer Ingelheim) (November-2017) Halimatoz (Sandoz) (July-2018) Hefiya (Sandoz) (July-2018) Hulio® (Mylan / Fujifilm Kyowa Kirin Biologics) (September-2018) Hyrimoz® (Sandoz) (July-2018 low-concentration, April-2023 high-concentration) Idacio® (Fresenius Kabi) (April-2019) Imraldi (Samsung Bioepis) (August-2017) Kromeya (Fresenius Kabi) (April-2019) Libmyris / Hukyndra (high and low-concentration) (Stada Arzneimittel AG / Alvotech) (November-2021)Yuflyma® (Celltrion) (February-2021 low-concentration, February-2022 high-concentration)
Biosimilars Approved In Australia
Abrilada (Pfizer) (February-2021) Amgevita (Amgen) (November-2017) Ciptunec / Ardalicip (high-concentration) (Alvotech / Cipla) (November-2022)Hadlima (Samsung Bioepis) (January-2018) Hulio (Alphapharm) (February-2021) Hyrimoz® (Sandoz) (May-2019) Idacio® (Fresenius Kabi) (June-2020) Yuflyma (Celltrion) (February-2022)
Biosimilars Approved In Japan
Adalimumab BS (Fujifilm Kyowa Kirin Biologics / Viatris / Sandoz) (June-2020) Adalimumab BS MA (LG Chem / Mochida Pharmaceutical) (March-2021)
Biosimilars Approved In South Korea
Adalloce (Samsung Bioepis) (September-2017, high-concentration January-2023) Xelenka (high-concentration) (LG Chem) (December-2023)Yuflyma® (high-concentration) (Celltrion) (October-2021)