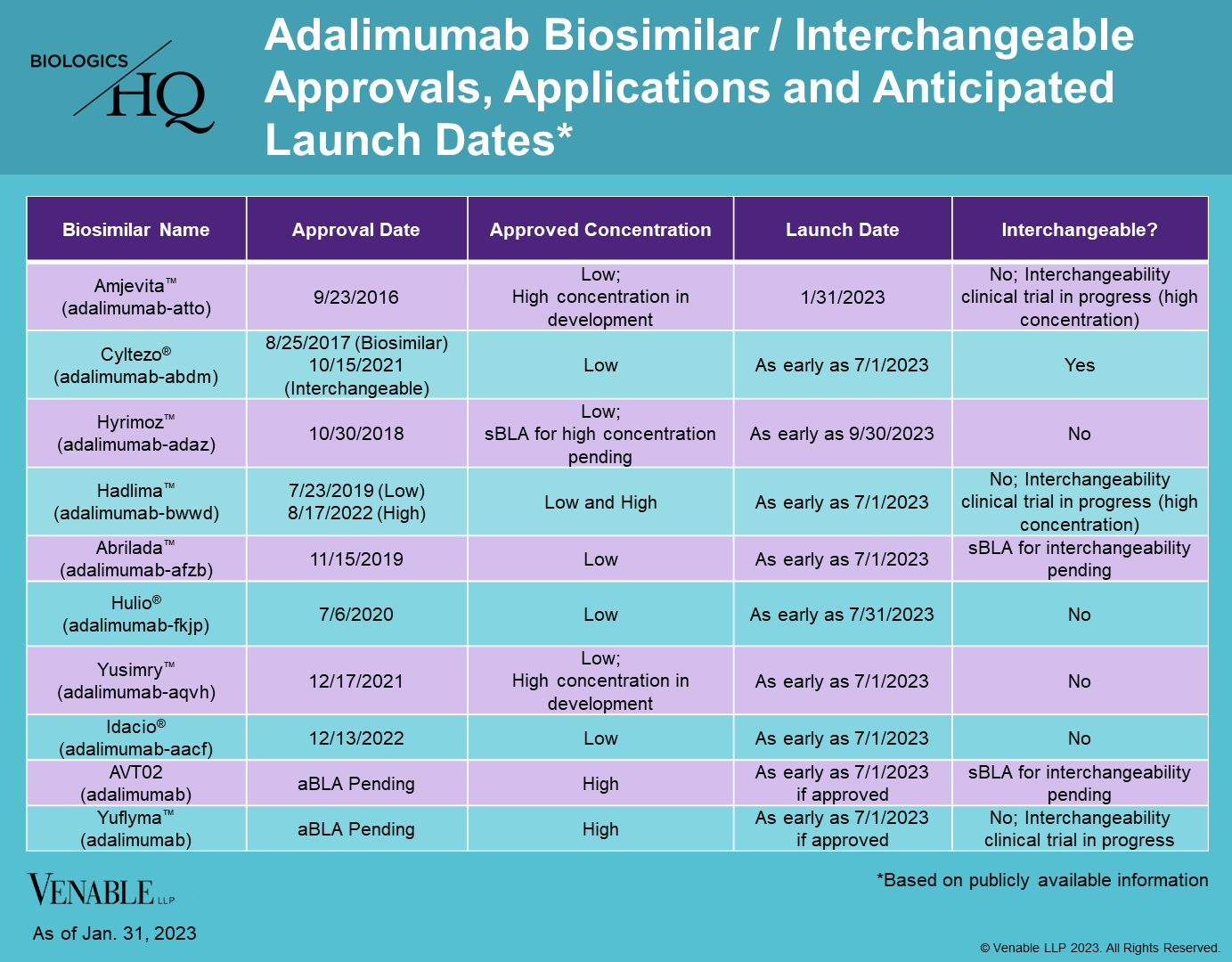
On January 31, 2023, Amjevita™ (adalimumab-atto) entered the U.S. market as the first biosimilar of Humira® (adalimumab), after receiving approval in September 2016. Amjevita will be available in a low-concentration, citrate-free formulation (40 mg/0.8mL and 20mg/0.4mL) in prefilled syringes and for autoinjectors. There will be two different pricing options, the first at a list price (wholesale acquisition cost) that is a 5% discount compared to Humira, and the other at a 55% discount. The various discounts are offered to accommodate different rebating and discounting practices by payers and pharmacy benefit managers.
Amjevita is the first Humira biosimilar to enter the U.S. market, with at least seven additional biosimilars likely to launch this year based on settlement agreements. Cyltezo® (adalimumab-adbm), the first biosimilar approved as interchangeable with Humira, with the possibility for automatic substitution at the pharmacy, will launch as early as July 1, 2023. Hadlima™ (adalimumab-bwwd), the first biosimilar approved as a high-concentration, citrate-free formulation, will be available as early as July 1, 2023. The high-concentration formulation of Humira accounts for over 80% of its prescriptions.