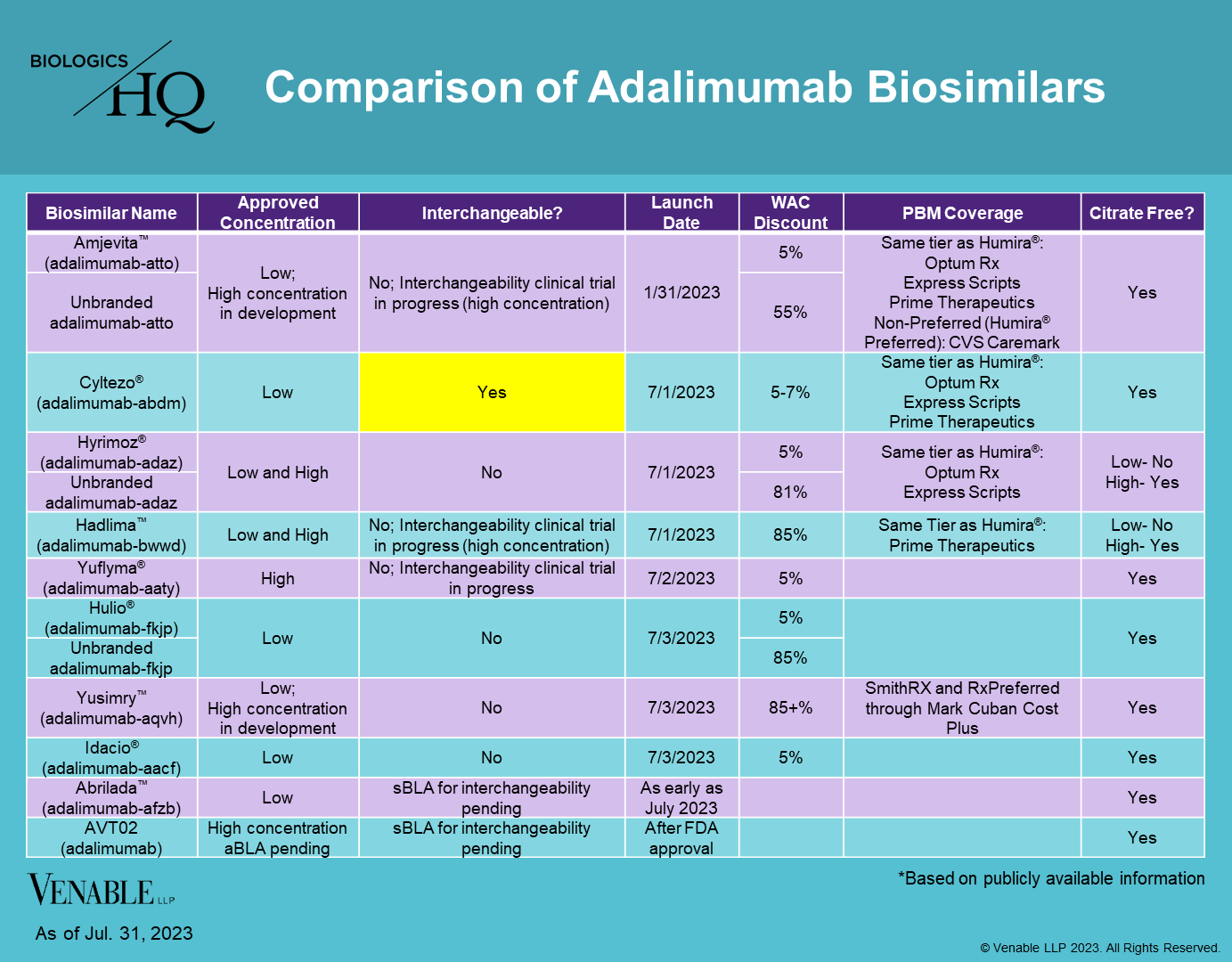
FDA Approved Biologics and Pending Applications
FDA Approved Biologics and Pending Applications
chorionic gonadotropin
BL 017067
denosumab-bnht
aBL 761398
rituximab
DRL_RI Approval Pending
adalimumab-aacf
aBL 761255
ustekinumab-aauz
aBL 761379
pegfilgrastim-fpgk
aBL 761173
tocilizumab-aazg
aBL 761275 / aBL 761449
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Idacio® (adalimumab) (Fresenius Kabi) (October-2020) Otulfi® (Fresenius Kabi) (ustekinumab) (December-2024)Tyenne® (tocilizumab) (Fresenius Kabi) (October-2024)
Biosimilars Approved In The E.U.
Idacio® (adalimumab) (Fresenius Kabi) (April-2019) Kromeya (adalimumab) (Fresenius Kabi) (April-2019) Otulfi® (Fresenius Kabi) (ustekinumab) (September-2024)Stimufend® (pegfilgrastim) (Fresenius Kabi) (March-2022)Tyenne® (tocilizumab) (Fresenius Kabi) (September-2023)
Biosimilars Approved In Australia
Idacio® (adalimumab) (Fresenius Kabi) (June-2020)
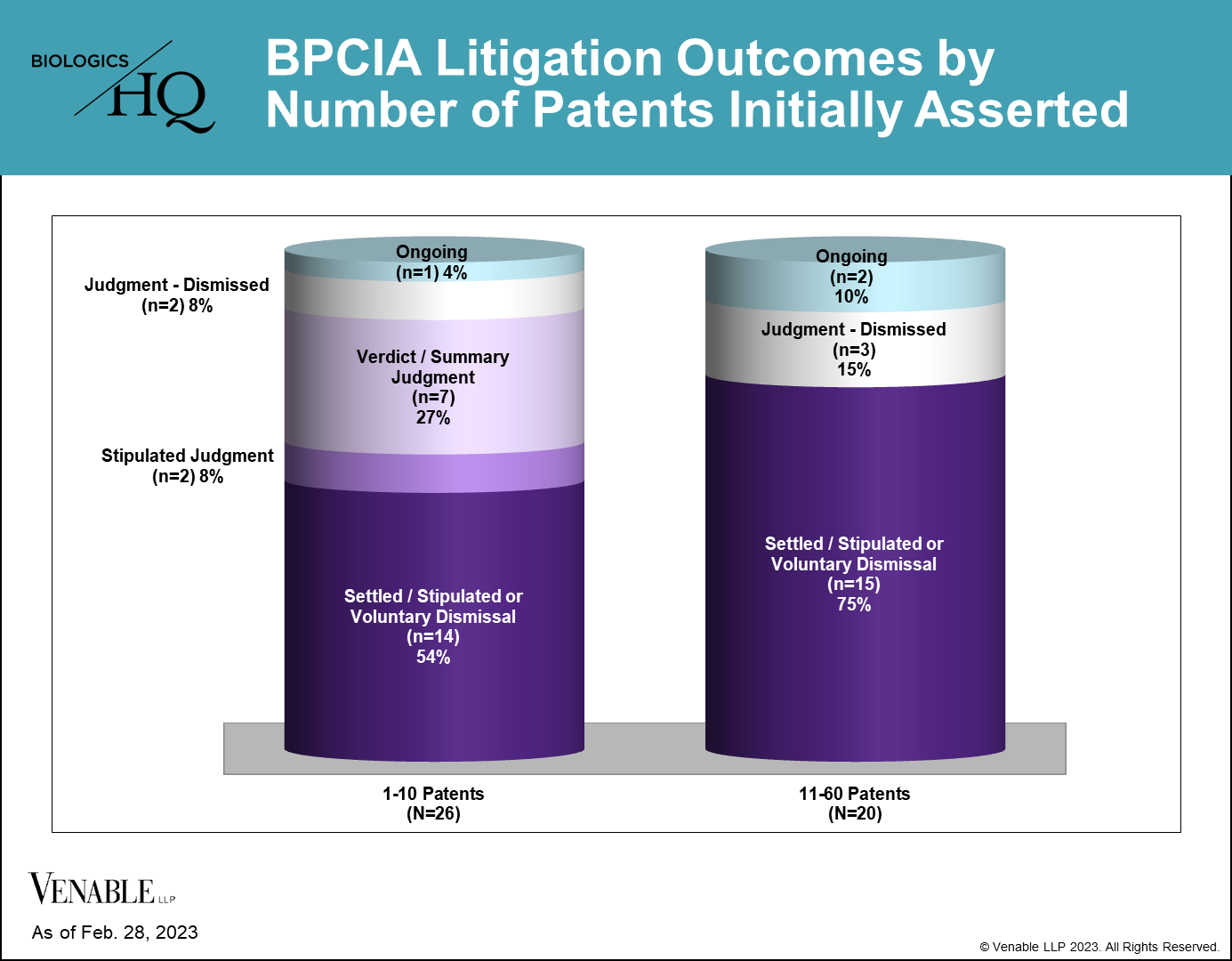
 U.S. Patent Litigations
U.S. Patent Litigations
PROLIA / XGEVA / CONEXXENCE / BOMYNTRA1:25-cv-01080 (D.N.J.) (transferred from 1:24-cv-09555 (N.D. Ill.)) / MDL 1:25-md-03138 (D.N.J.)RITUXAN / DRL_RI1:23-cv-22485 (D.N.J.)