FDA Approved Biologics and Pending Applications
FDA Approved Biologics and Pending Applications
ranibizumab-nuna
aBL 761202
eculizumab-aagh
aBL 761340
etanercept-ykro
aBL 761066
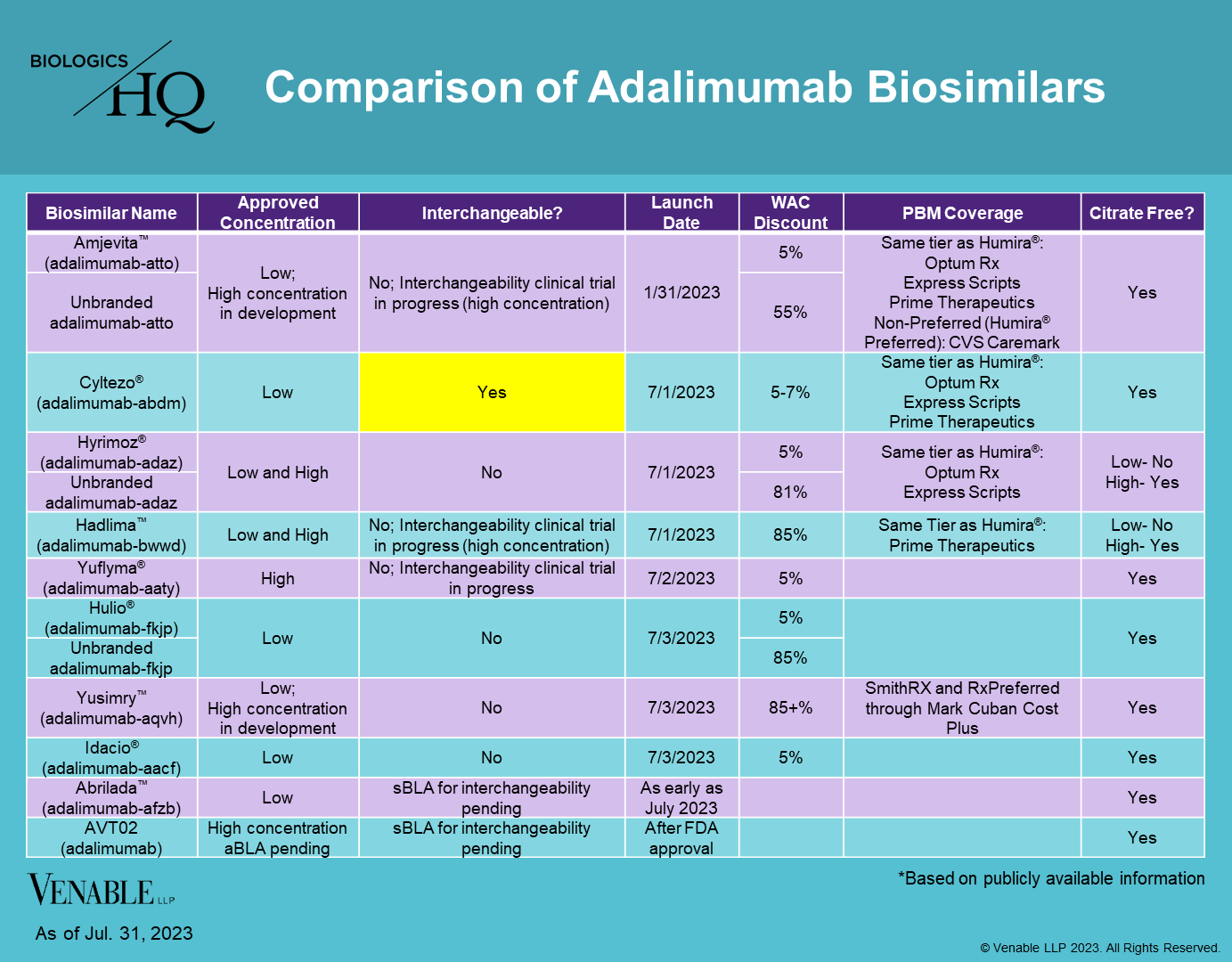
adalimumab-bwwd
aBL 761059
trastuzumab-dttb
aBL 761100
aflibercept-yszy
aBL 761350
denosumab-dssb
aBL 761392
ustekinumab-ttwe
aBL 761373 / aBL 761425
infliximab-abda
BL 761054
bevacizumab
SB8 Approval Pending
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Aybintio (bevacizumab) (Samsung Bioepis) (November-2021)Brenzys (etanercept) (Samsung Bioepis / Merck Canada) (August-2016)Byooviz (ranibizumab) (Samsung Bioepis / Biogen) (March-2022)Hadlima (Samsung Bioepis) (May-2018, high-concentration January-2023)Ontruzant® (trastuzumab) (Samsung Bioepis) (February-2022)Opuviz® (aflibercept) (Samsung Bioepis) (October-2025)Renflexis (infliximab) (Samsung Bioepis) (March-2018)
Biosimilars Approved In The E.U.
Aybintio (bevacizumab) (Samsung Bioepis) (August-2020) Benepali (etanercept) (Samsung Bioepis / Biogen) (January-2016)Byooviz (ranibizumab) (Samsung Bioepis / Biogen) (August-2021)Eksunbi (ustekinumab) (Samsung Bioepis) (September-2024)Epysqli (eculizumab) (Samsung Bioepis) (May-2023)Flixabi (infliximab) (Samsung Bioepsis) (May-2016)Imraldi (adalimumab) (Samsung Bioepis) (August-2017)Obodence / Xbryk (denosumab) (Samsung Bioepis) (February-2025)Onbevzi (bevacizumab) (Samsung Bioepis) (January-2021)Ontruzant® (trastuzumab) (Samsung Bioepis) (November-2017)Opuviz® (aflibercept) (Samsung Bioepis / Biogen) (November-2024)Pyzchiva® (ustekinumab) (Sandoz / Samsung Bioepis) (April-2024)
Biosimilars Approved In Australia
Brenzys (etanercept) (Samsung Bioepis) (July-2016)Byooviz (ranibizumab) (Samsung Bioepis) (August-2022)Hadlima (adalimumab) (Samsung Bioepis) (January-2018)Onbevzi® (bevacizumab) (Samsung Bioepis) (January-2024)Ontruzant® (trastuzumab) (Samsung Bioepis) (January-2019) Renflexis (infliximab) (Samsung Bioepis / Merck) (November-2016)
Biosimilars Approved In Japan
Ustekinumab BS NIPRO (ustekinumab) (Samsung Bioepis) (December-2025)
Biosimilars Approved In South Korea
Adalloce (adalimumab) (Samsung Bioepis) (September-2017, high-concentration January-2023)Afilivu (aflibercept) (Samsung Bioepis / Samil) (February-2024)Amelivu (ranibizumab) (Samsung Bioepis) (May-2022)Epysqli (eculizumab) (Samsung Bioepis) (January-2024)Epyztek (ustekinumab) (Samsung Bioepis) (April-2024)Etoloce (etanercept) (Samsung Bioepis) (September-2015)Obodence (denosumab) (Samsung Bioepis) (April-2025) Onbevzi® (bevacizumab) (Boryung Pharmaceutical / Samsung Bioepis) (March-2021)Remaloce (infliximab) (Samsung Bioepis / Merck) (December-2015)Samfenet (trastuzumab) (Samsung Bioepis) (November-2017)Xbryk (denosumab) (Samsung Bioepis) (May-2025)Xbryk (denosumab) (Samsung Bioepis) (May-2025)Xbryk (denosumab) (Samsung Bioepis) (May-2025)Xbryk (denosumab) (Samsung Bioepis) (May-2025)Xbryk (denosumab) (Samsung Bioepis) (May-2025)Xbryk (denosumab) (Samsung Bioepis) (May-2025)Xbryk (denosumab) (Samsung Bioepis) (May-2025)
 Inter Partes Review Proceedings
Inter Partes Review Proceedings
EYLEA / OPUVIZIPR2023-00442IPR2023-00566IPR2023-00739IPR2023-00884IPR2023-01312IPR2025-00176HERCEPTIN / ONTRUZANTIPR2017-01958IPR2017-01959IPR2017-01960IPR2017-02139IPR2017-02140IPR2018-00192SOLIRIS / EPYSQLIIPR2023-00933IPR2023-00998IPR2023-00999IPR2023-01069IPR2023-01070STELARA / PYZCHIVAIPR2023-01103
 U.S. Patent Litigations
U.S. Patent Litigations
AVASTIN / SB81:20-cv-00859 (D. Del.)ENBREL / ETICOVO2:19-cv-11755 (D.N.J.) EYLEA / OPUVIZ1:23-cv-00094 (N.D.W. Va.) / MDL 1:24-md-03103 (N.D.W. Va.)1:23-cv-00106 (N.D.W. Va.) / MDL 1:24-md-03103 (N.D.W. Va.)HERCEPTIN / ONTRUZANT / GENENTECH CABILLY1:18-cv-01363 (D. Del.)PROLIA / XGEVA / OSPOMYV / XBRYK1:24-cv-08417 (D.N.J.) / MDL 1:25-md-03138 (D.N.J.)REMICADE / RENFLEXIS2:17-cv-03524 (D.N.J.)SOLIRIS / EPYSQLI1:24-cv-00005 (D. Del.)