FDA Approved Biologics and Pending Applications
FDA Approved Biologics and Pending Applications
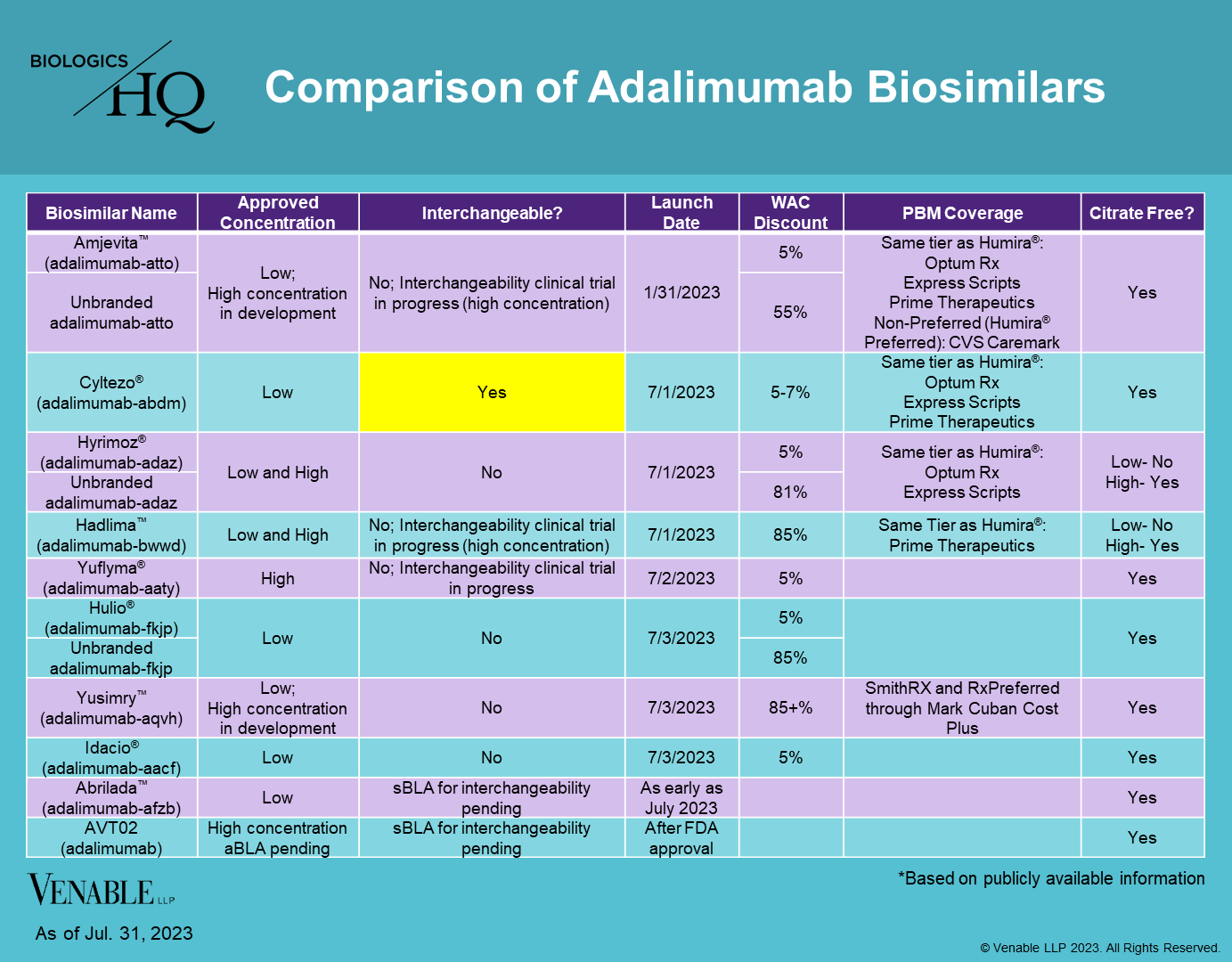
adalimumab-afzb
aBL 761118
fidanacogene elaparvovec-dzkt
BL 125786
taliglucerase alfa
BL 022458
elranatamab
BL 761345
somatropin
BL 020280
marstacimab-hncq
BL 761369
Infliximab-dyyb
aBL 125544
infliximab-qbtx
aBL 761072
somatrogon-ghla
BL 761184
filgrastim-aafi
aBL 761080
pegfilgrastim-apgf
aBL 761111
epoetin alfa-epbx
aBL 125545
rituximab-pvvr
aBL 761103
pegvisomant
BL 021106
trastuzumab-qyyp
aBL 761081
bevacizumab-bvzr
aBL 761099
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Adalimumab Injection (adalimumab) (Pfizer Canada) (February-2021)Ixifi (infliximab) (Pfizer) (December-2021)Nivestym® (filgrastim) (Pfizer) (April-2020)Ruxience® (rituximab) (Pfizer) (May-2020)Trazimera® (trastuzumab) (Pfizer) (August-2019)Zirabev® (bevacizumab) (Pfizer) (June-2019)
Biosimilars Approved In The E.U.
Amsparity (adalimumab) (Pfizer) (February-2020)Nyvepria® (pegfilgrastim) (Pfizer) (November-2020)Ruxience® (rituximab) (Pfizer) (April-2020)Trazimera® (trastuzumab) (Pfizer) (July-2018)Zirabev® (bevacizumab) (Pfizer) (February-2019)
Biosimilars Approved In Australia
Abrilada (adalimumab) (Pfizer) (February-2021)Nivestim® (filgrastim) (Pfizer) (September-2010)Ruxience® (rituximab) (Pfizer) (March-2021)Trazimera® (trastuzumab) (Pfizer) (August-2019)Zirabev® (bevacizumab) (Pfizer) (November-2019)
Biosimilars Approved In Japan
Infliximab BS 3 (infliximab) (Pfizer) (July-2018)Rituximab BS (rituximab) (Pfizer) (September-2019)
Biosimilars Approved In South Korea
Zirabev® (bevacizumab) (Pfizer) (May-2021)
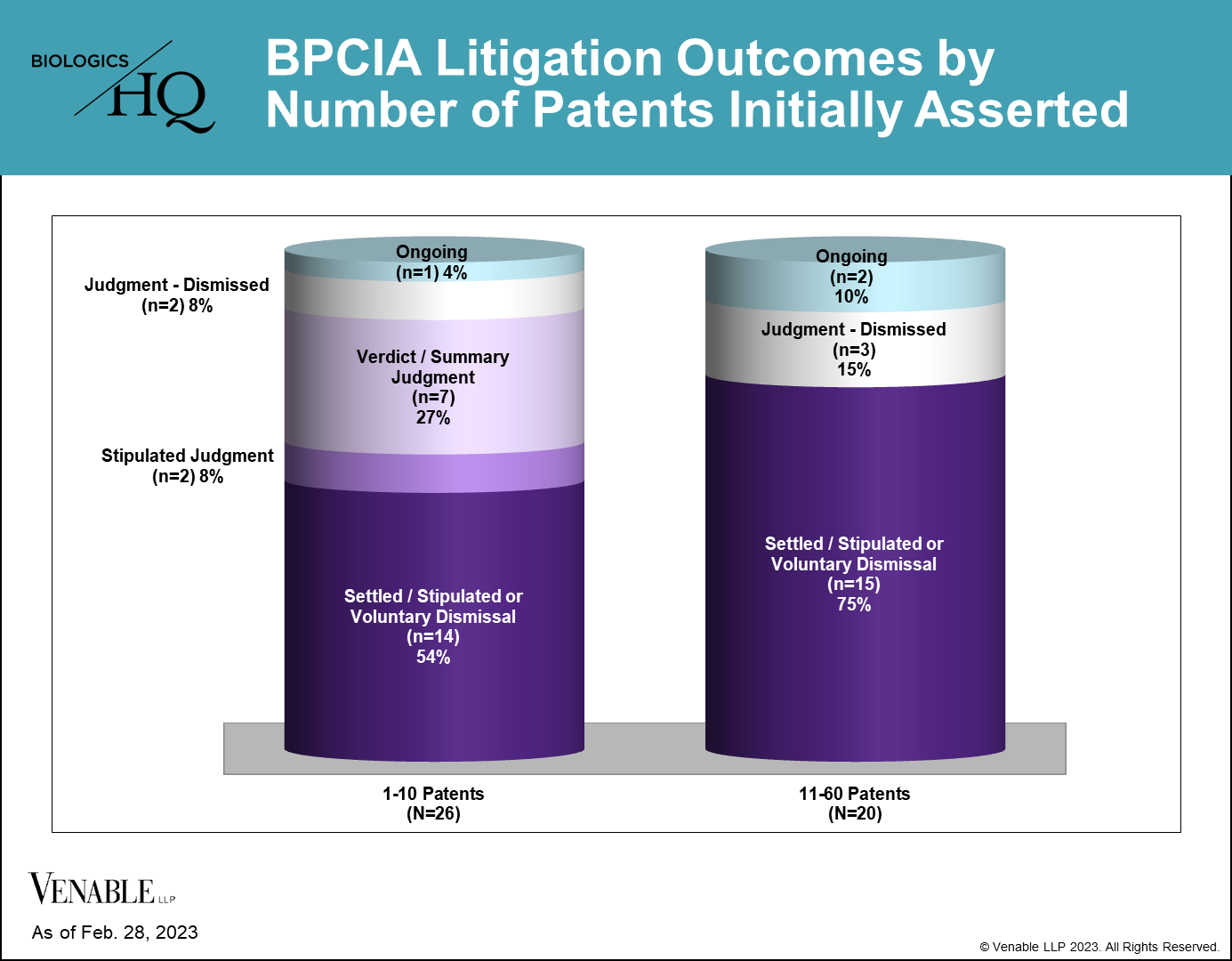
 Inter Partes Review Proceedings
Inter Partes Review Proceedings
HEMGENIXIPR2020-00388IPR2021-00925IPR2021-00926IPR2021-00928LANTUS / LANTUS SOLOSTARIPR2019-00977IPR2019-00978IPR2019-00979IPR2019-00980IPR2019-00981IPR2019-00982IPR2019-00987IPR2019-01022IPR2019-01023AVASTIN / ZIRABEVIPR2016-01771IPR2018-00373HERCEPTIN / TRAZIMERAIPR2017-00731IPR2017-00737IPR2017-00739IPR2017-00804IPR2017-00805IPR2017-01488IPR2017-01489IPR2017-01726IPR2017-01727IPR2017-02019IPR2017-02020IPR2017-02063IPR2018-00016IPR2018-00330IPR2018-00331NEUPOGEN / NEULASTA / NIVESTYM / NYVEPRIAIPR2021-00528RITUXAN / RUXIENCEIPR2017-01115IPR2017-01166IPR2017-01167IPR2017-01168IPR2017-01923IPR2017-02126IPR2017-02127IPR2018-00086IPR2018-00186IPR2018-00231IPR2018-00285
 U.S. Patent Litigations
U.S. Patent Litigations
AVASTIN / ZIRABEV1:19-cv-00638 (D. Del.)AVONEX / BETASERON / EXTAVIA / REBIF2:10-cv-02734 (D.N.J.)2:10-cv-02760 (D.N.J.)HERCEPTIN / TRAZIMERA / GENENTECH CABILLY1:17-cv-01672 (D. Del.)NEULASTA / NYVEPRIA1:20-cv-00201 (D. Del.)NEUPOGEN / NIVESTYM1:18-cv-01064 (D. Del.)1:20-cv-00561 (D. Del.)OPDIVO / BAVENCIO1:17-cv-01029 (D. Del.)