Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
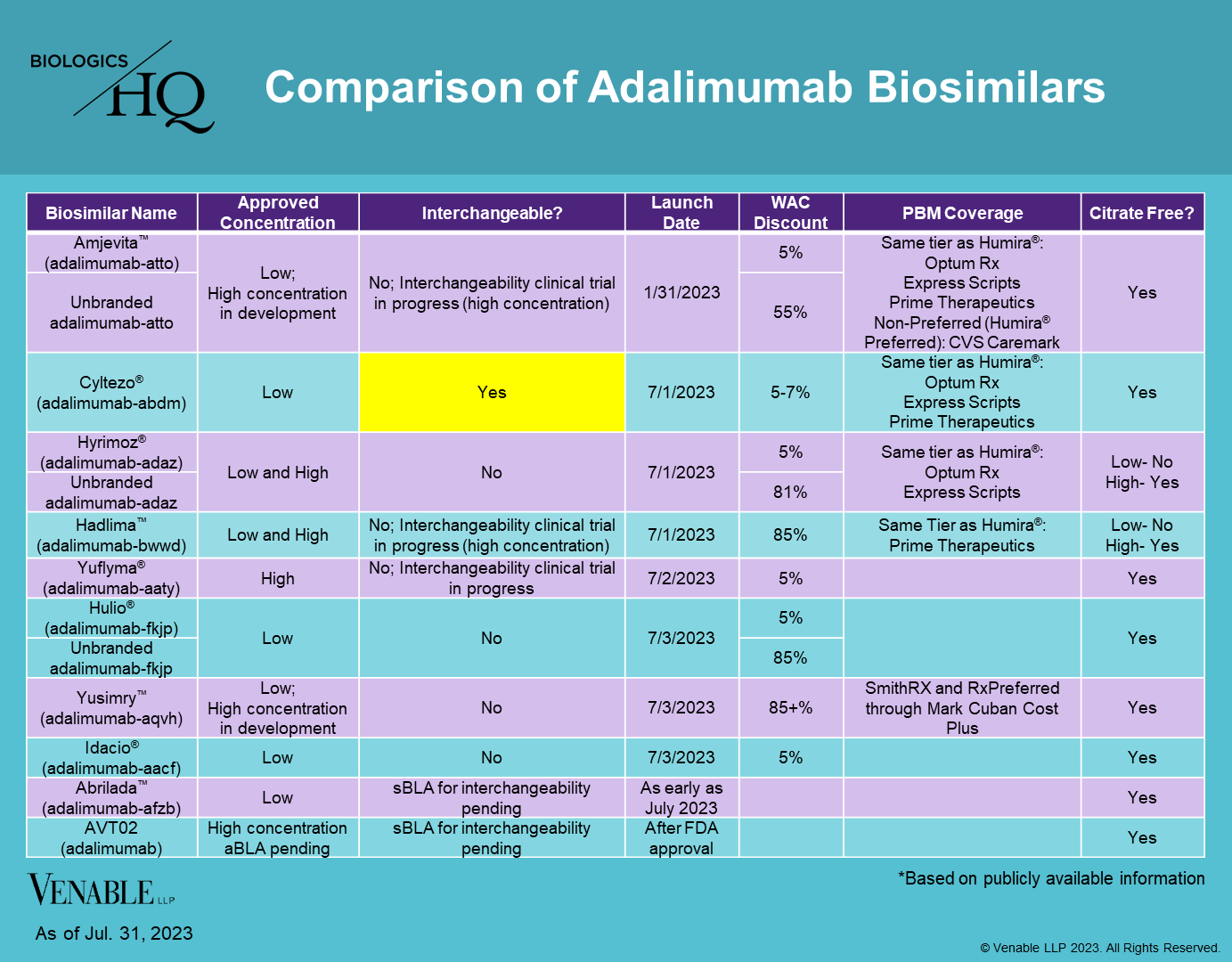
Biosimilars Approved In The E.U.
Abasaglar (insulin glargine recombinant) (Eli Lilly / Boehringer Ingelheim) (September-2014)Cyltezo (adalimumab) (Boehringer Ingelheim) (November-2017)
Biosimilars Approved In Japan
Insulin glargine BS (insulin glargine recombinant) (Eli Lilly / Boehringer Ingelheim) (December-2014)