FDA Approved Biologics and Pending Applications
FDA Approved Biologics and Pending Applications
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Fulphila® (pegfilgrastim) (Mylan / Biocon) (May-2019) Ogivri® (trastuzumab) (Mylan / Biocon) (May-2019)
Biosimilars Approved In The E.U.
Fulphila® (pegfilgrastim) (Mylan / Biocon) (November-2018)Hulio® (adalimumab) (Mylan / Fujifilm Kyowa Kirin Biologics) (September-2018)Nepexto (etanercept) (Mylan / Lupin) (June-2020)Ogivri® (trastuzumab) (Mylan / Biocon) (December-2018)Semglee® (insulin glargine) (Mylan / Biocon) (March-2018)
Biosimilars Approved In Australia
Fulphila® (pegfilgrastim) (Alphapharm / Mylan / Biocon) (August-2018)Ogivri® (trastuzumab) (Mylan / Biocon) (December-2018) Semglee® (insulin glargine) (Mylan / Biocon) (March-2018)
Biosimilars Approved In Japan
Adalimumab BS FKB (adalimumab) (Fujifilm Kyowa Kirin Biologics / Viatris / Sandoz) (June-2020)
Biosimilars Approved In South Korea
Ogivri® (trastuzumab) (Alvogen Korea) (August-2020)
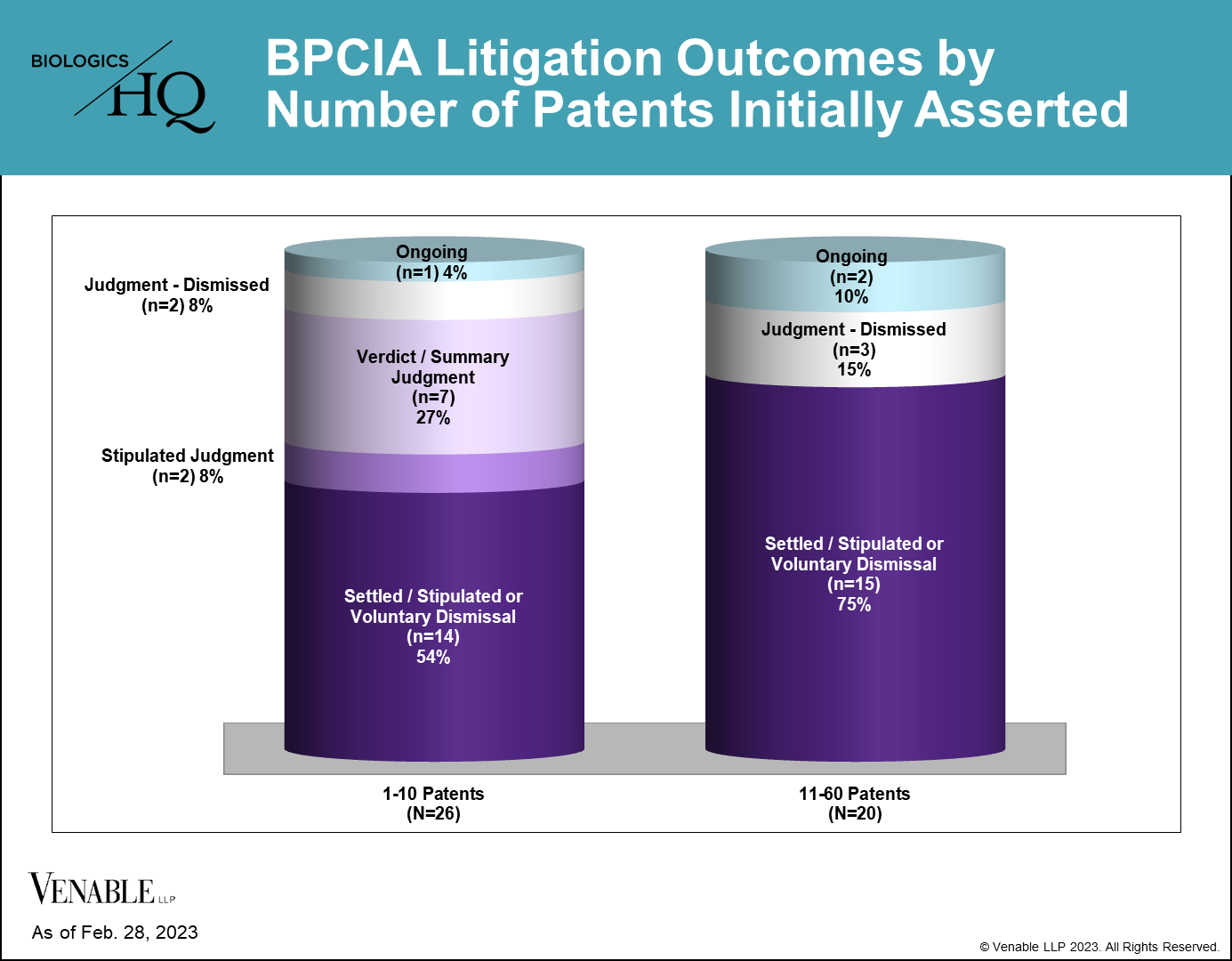
 Inter Partes Review Proceedings
Inter Partes Review Proceedings
GENENTECH CABILLYIPR2016-00710ORENCIAIPR2015-01537EYLEA / YESAFILIIPR2021-00880IPR2021-00881IPR2022-01225IPR2022-01226IPR2023-00099IPR2024-00201IPR2024-00298HERCEPTIN / OGIVRIIPR2016-01693IPR2016-01694LANTUS / LANTUS SOLOSTAR / SEMGLEEIPR2017-01526IPR2017-01528IPR2018-01670IPR2018-01675IPR2018-01676IPR2018-01677IPR2018-01678IPR2018-01679IPR2018-01680IPR2018-01682IPR2018-01684IPR2018-01696IPR2019-00122IPR2019-01657IPR2019-01658