FDA Approved Biologics and Pending Applications
FDA Approved Biologics and Pending Applications
bevacizumab-tnjn
aBL 761198
ranibizumab-eqrn
aBL 761165
trastuzumab
EG12014 Approval Pending
aflibercept-abzv
aBL 761382
etanercept-szzs
aBL 761042
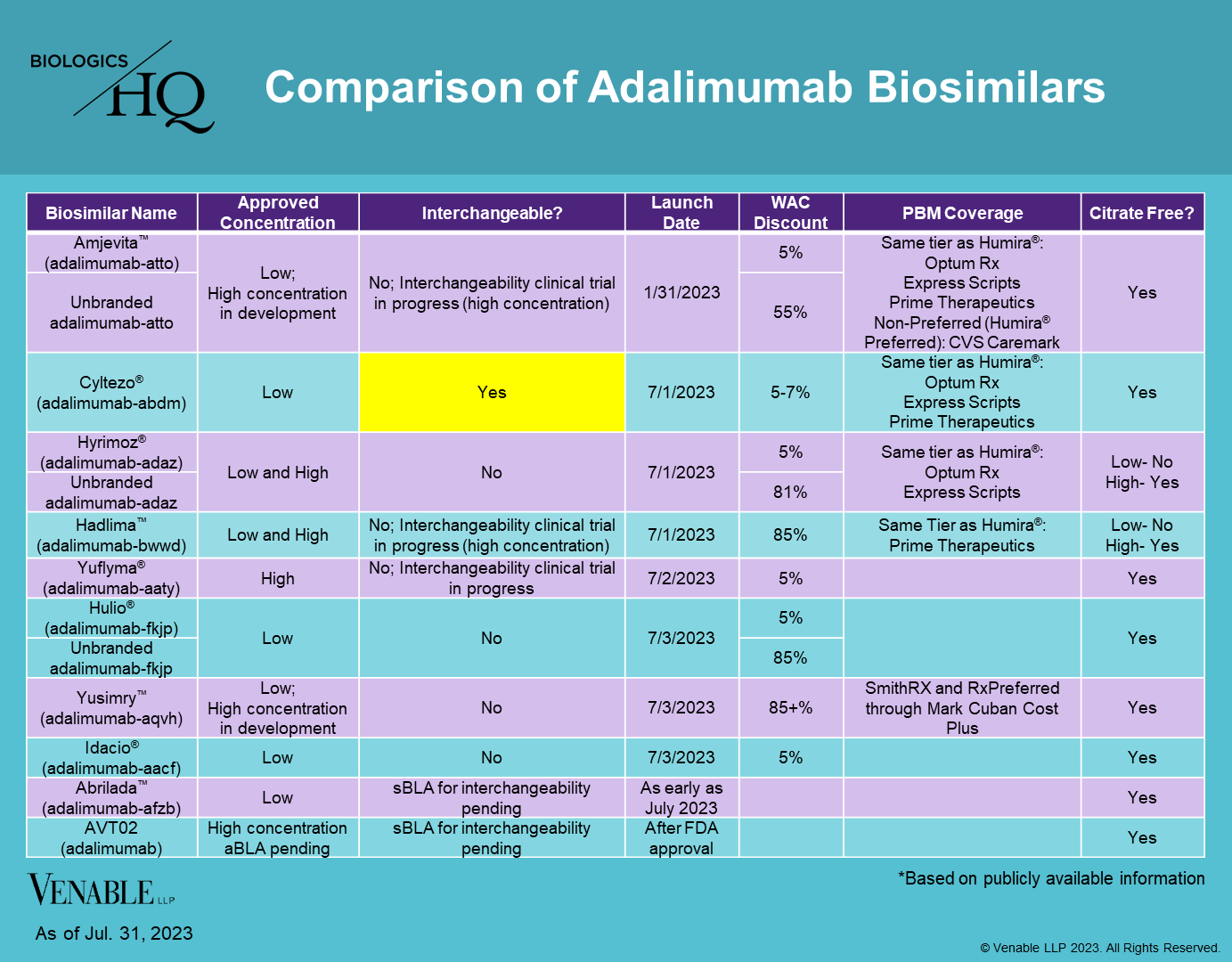
adalimumab-adaz
aBL 761071
denosumab-bbdz
aBL 761362
somatropin
aBL 021426
ustekinumab-ttwe
aBL 761373 / aBL 761425
rituximab
Rixathon® Approval Pending
natalizumab-sztn
aBL 761322
filgrastim-sndz
aBL 125553
pegfilgrastim-bmez
aBL 761045
 U.S. Patent Litigations
U.S. Patent Litigations
ENBREL / ERELZI2:16-cv-01118 (D.N.J.) 3:13-cv-02904 (N.D. Cal.) ENBREL / ETICOVO2:19-cv-11755 (D.N.J.) EYLEA / ENZEEVU1:24-cv-00085 (N.D.W. Va.) (transferred from 2:24-cv-08760 (D.N.J.)) / MDL 1:24-md-03103 (N.D.W. Va.)HUMIRA / HYRIMOZ3:18-cv-12668 (D.N.J.)NEULASTA / ZIEXTENZO2:16-cv-01276 (D.N.J.)3:16-cv-02581 (N.D. Cal.)3:19-cv-00977 (N.D. Cal.) NEUPOGEN / ZARXIO3:14-cv-04741 (N.D. Cal.) 3:19-cv-00977 (N.D. Cal.) NEUPOGEN / ZARXIO / NEULASTA / ZIEXTENZO3:19-cv-00977 (N.D. Cal.) PROLIA / XGEVA / JUBBONTI / WYOST1:23-cv-02406 (D.N.J.)RITUXAN / RIXATHON / GENENTECH CABILLY1:17-cv-13507 (D.N.J.)TYSABRI / TYRUKO1:22-cv-01190 (D. Del.)