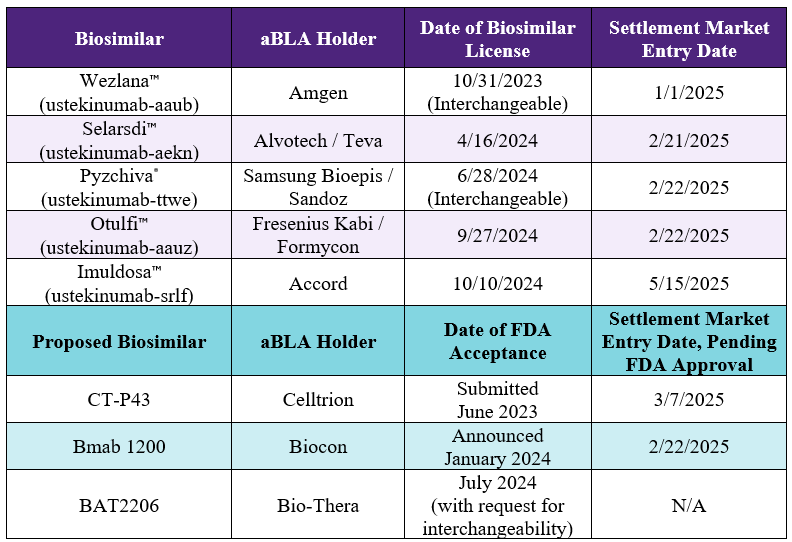
FDA Approved Biologics and Pending Applications
FDA Approved Biologics and Pending Applications
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Imuldosa® (ustekinumab) (Dong-A / Accord) (January-2026)Yesintek (ustekinumab) (Biocon) (October-2025)
Biosimilars Approved In The E.U.
Accofil® (filgrastim) (Accord Healthcare) (September-2014)Imuldosa® / Absimky (ustekinumab) (Accord) (December-2024)Osvyrti® / Jubereq® (denosumab) (Accord) (May-2025)Pelgraz (pegfilgrastim) (Accord Healthcare) (September-2018)Sondelbay (teriparatide) (Accord Healthcare) (March-2022)Yesintek (ustekinumab) (Biocon) (February-2025 EU, May-2025 UK)Zercepac (trastuzumab) (Shanghai Henlius Biotech / Accord Healthcare Ltd.) (July-2020)
Biosimilars Approved In Australia
Neutropeg® (pegfilgrastim) (Accord Healthcare) (August-2019) Pelgraz® (pegfilgrastim) (Accord Healthcare) (December-2019)
Biosimilars Approved In Japan
Ustekinumab BS YD (ustekinumab) (Biocon / Yoshindo) (December-2024)
 U.S. Patent Litigations
U.S. Patent Litigations
NEUPOGEN / NEULASTA / GRASTOFIL / LAPELGA0:18-cv-61828 (S.D. Fla.)PROLIA / XGEVA / OSVYRTI / JUBEREQ1:25-cv-01305 (D.N.J.) (transferred from 5:24-cv-00642 (E.D.N.C.)) / MDL 1:25-md-03138 (D.N.J.)