U.S. License Holder:
Bio-Thera Solutions
Date of License:
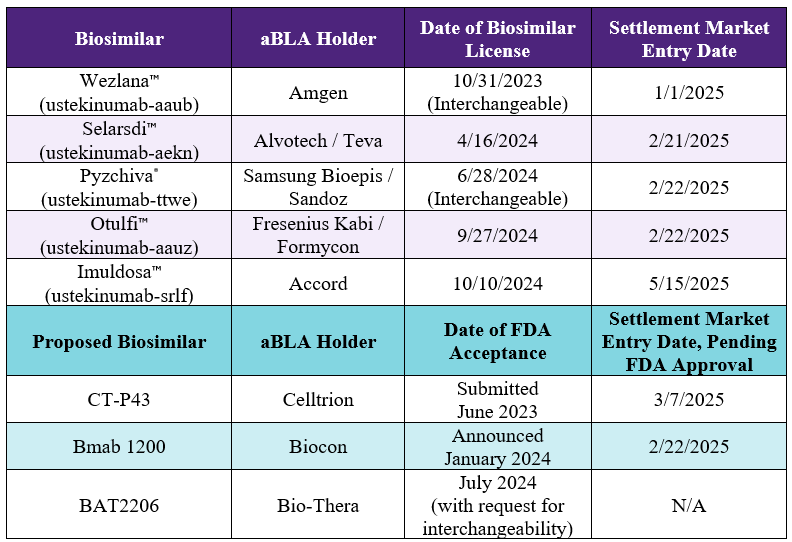
aBLA accepted by FDA July-2024
Last Update:
May-15-2025
 FDA-Approved Indications
FDA-Approved Indications
BAT2206 (ustekinumab) is not FDA-approved. An aBLA has been accepted by the FDA.