U.S. License Holder:
Fresenius Kabi / Formycon
Date of License:
September-27-2024
Last Update:
Dec-15-2024
 FDA-Approved Indications
FDA-Approved Indications
OTULFI (ustekinumab-aauz) is a human interleukin -12 and -23 antagonist indicated for the treatment of:
Adult patients with:
Moderate to severe plaque psoriasis (PsO) who are candidates for phototherapy or systemic therapy;
Active psoriatic arthritis (PsA);
Moderately to severely active Crohn's disease (CD);
Moderately to severely active ulcerative colitis.
Pediatric patients 6 years and older with:
Moderate to severe plaque psoriasis, who are candidates for phototherapy or systemic therapy;
Active psoriatic arthritis (PsA).
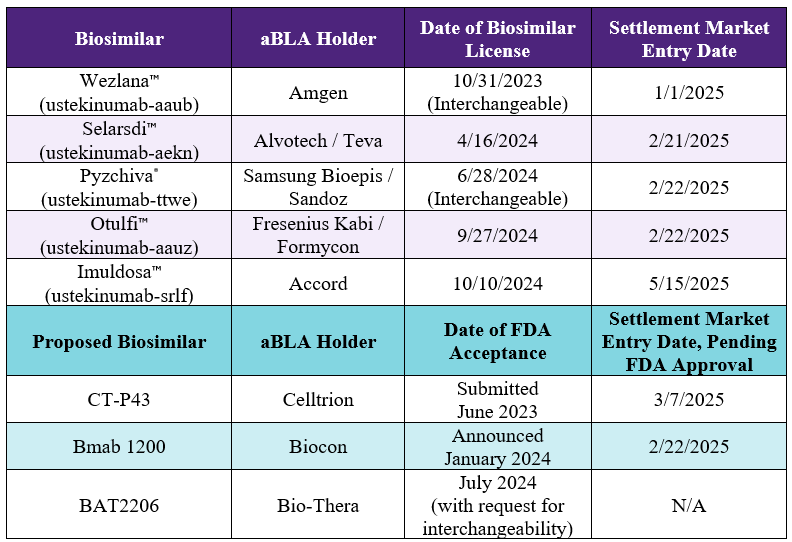
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In The E.U.
Fymskina (Formycon) (September-2024)Otulfi (Fresenius Kabi) (Septenber-2024)