U.S. License Holder:
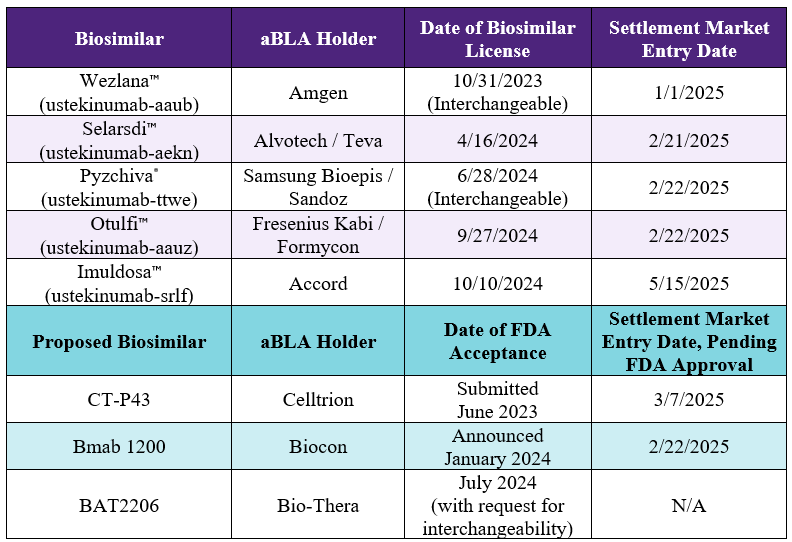
Alvotech / Teva
Date of License:
April-16-2024
Last Update:
Dec-15-2024
 FDA-Approved Indications
FDA-Approved Indications
SELARSDI (ustekinumab-aekn) is a human interleukin-12 and -23 antagonist indicated for the treatment of:
Adult patients with:
Moderate to severe plaque psoriasis (Ps) who are candidates for phototherapy or systemic therapy;
Active psoriatic arthritis (PsA)
Moderately to severely active Crohn's disease (CD);
Moderately to severely active ulcerative colitis.
Pediatric patients 6 years and older with:
Moderate to severe plaque psoriasis, who are candidates for phototherapy or systemic therapy;
Active psoriatic arthritis (PsA).
 Approved Foreign Follow-On Biologics / Biosimilars
Approved Foreign Follow-On Biologics / Biosimilars
Biosimilars Approved In Canada
Jamteki (Alvotech / JAMP Pharma) (November-2023)
Biosimilars Approved In The E.U.
Uzpruvo® (Alvotech / STADA Arzneimittel) (January-2024)
Biosimilars Approved In Japan
AVT04 (Alvotech / Fuji Pharma) (September-2023)