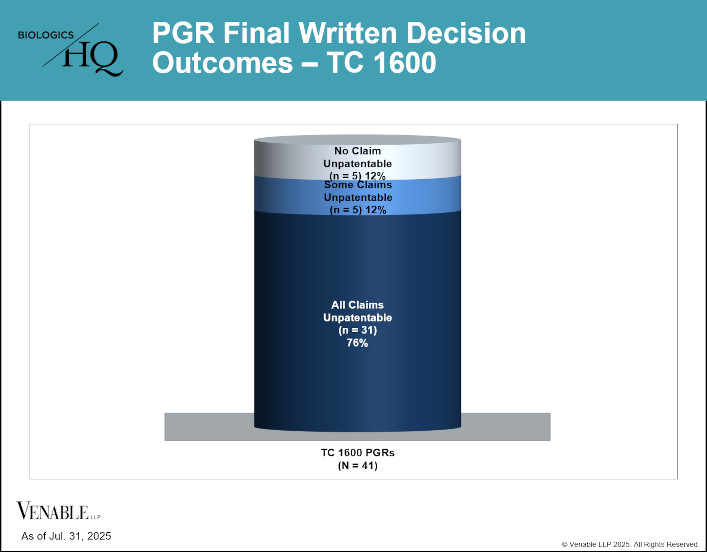
by April Breyer Menon | Aug 8, 2025
BiologicsHQ and materials published on BiologicsHQ are published for informational purposes only. Neither the information nor any opinion expressed on BiologicsHQ constitute legal advice, create an attorney-client relationship, or constitute a solicitation for...
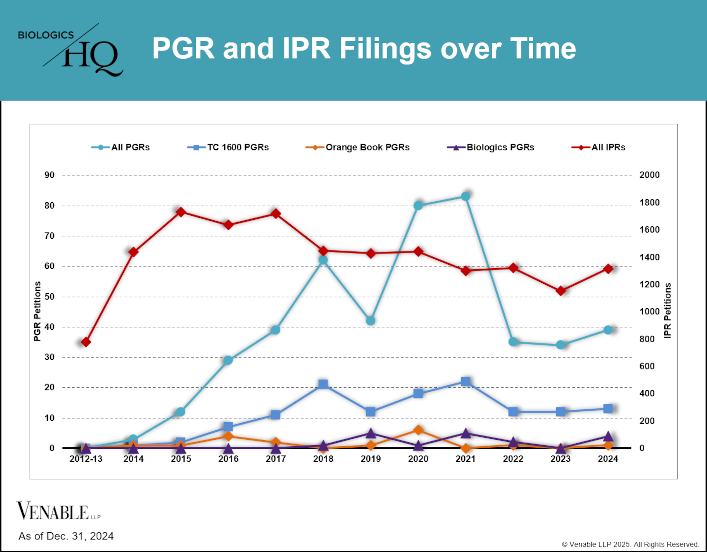
by April Breyer Menon | Feb 13, 2025
This figure shows the number of PGR and IPR petitions filed since their inception in 2012 through December 31, 2024. For our analysis, we reviewed PGR filings for all patent technologies (teal line) as well as the subsets of PGRs filed on Orange Book-listed...
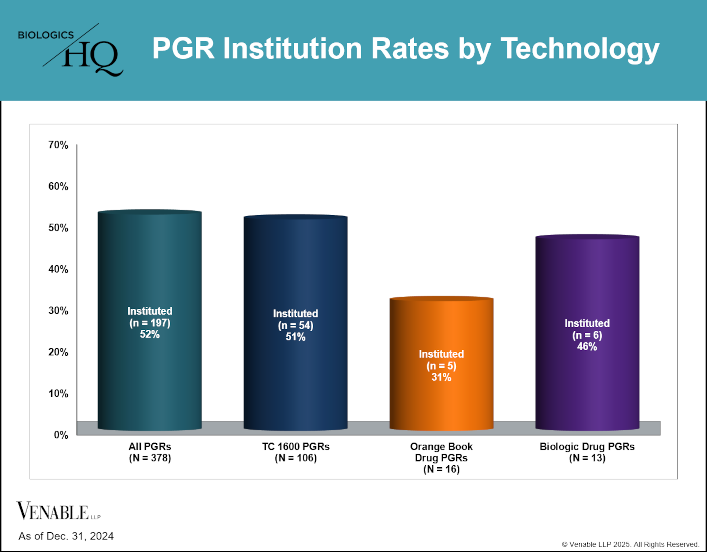
by April Breyer Menon | Feb 13, 2025
This figure shows PGR institution rates by technology. Within this relatively small data set, PGR institution rates varied, with TC 1600 patent challenges being instituted at a similar rate compared to that for all PGR institutions, while Orange Book-listed...
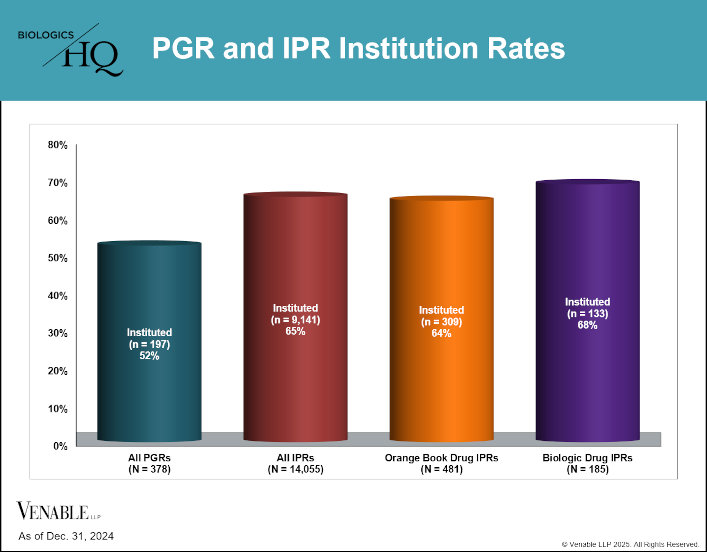
by April Breyer Menon | Feb 13, 2025
This figure shows institution rates for PGRs compared to IPRs[1] for all technologies as well as Orange Book and biologic drug IPRs. It is noteworthy that compared to the 52% institution rate for PGRs, IPRs have been instituted at a higher rate (65%). This...

by April Breyer Menon | Feb 13, 2025
This figure shows PGR and IPR institution rates over time.[1] PGR institution rates (teal line) were generally trending lower from their inception through FY 2021, but increased in FY 2022 and FY 2023 and surpassed IPRs in FY 2023 for the first time since FY...







