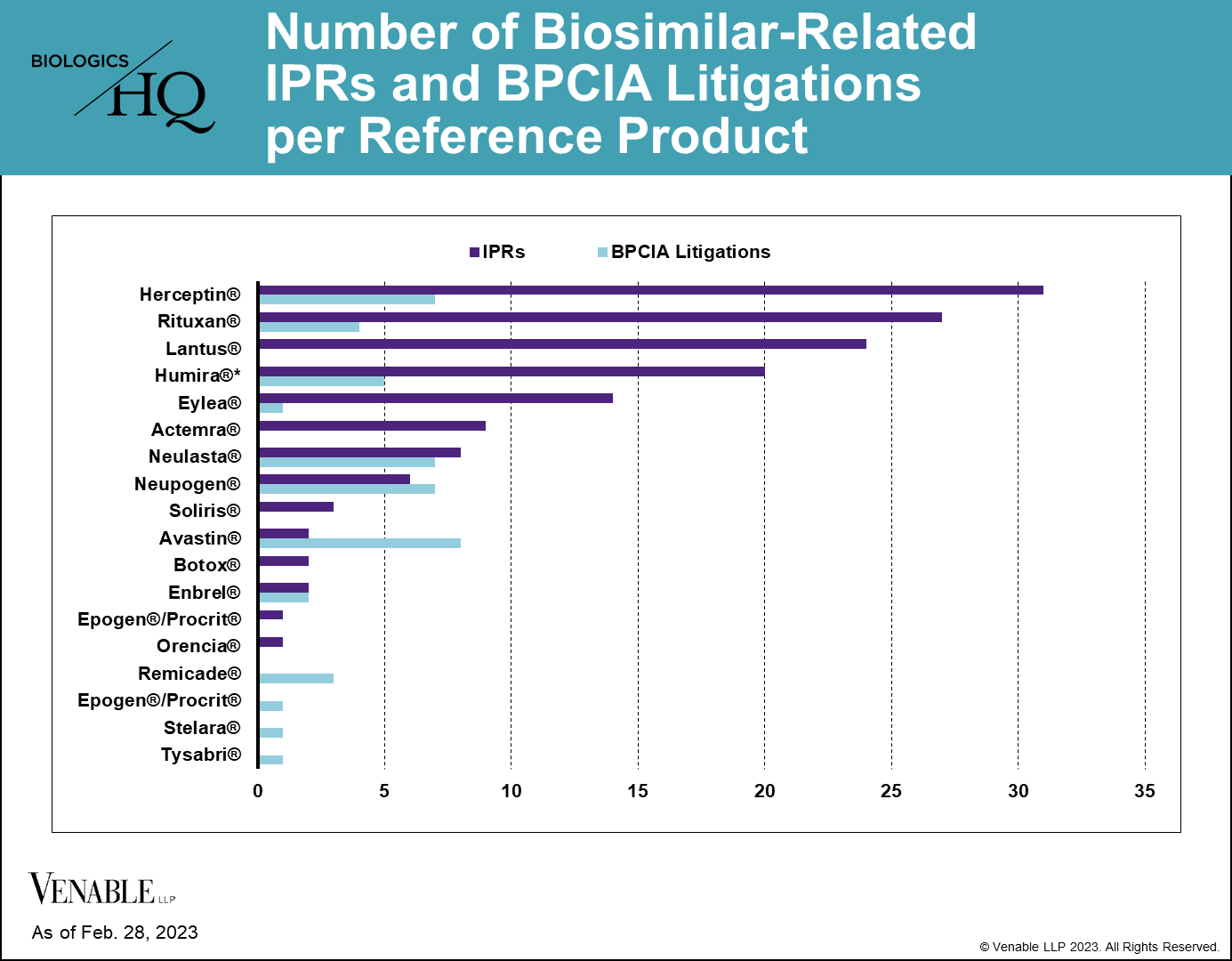
*One litigation is counted twice as it related to patents covering both Neupogen and Neulasta. While Lantus patents have been subject to litigation, none of those litigations were under the BPCIA and thus are not included.
As of February 28, 2023, there have been 144 biosimilar-related* IPRs encompassing 70 patents and 14 reference products, and there have been 46 BPCIA litigations related to 12 reference products. PGRs have not been a preferred way of resolving biosimilar-related patent disputes, with only three biosimilar-related PGR filings to date. While most of the RPSs have faced both IPRs and litigations related to their patents, some biologic patents have only faced one type of challenge, which can be seen in this graph.
*We define “biosimilar-related” patent disputes as those involving drug companies that have an approved biosimilar of the reference product or one in development at the time the dispute was filed.
BiologicsHQ and materials published on BiologicsHQ are published for informational purposes only. Neither the information nor any opinion expressed on BiologicsHQ constitute legal advice, create an attorney-client relationship, or constitute a solicitation for business.