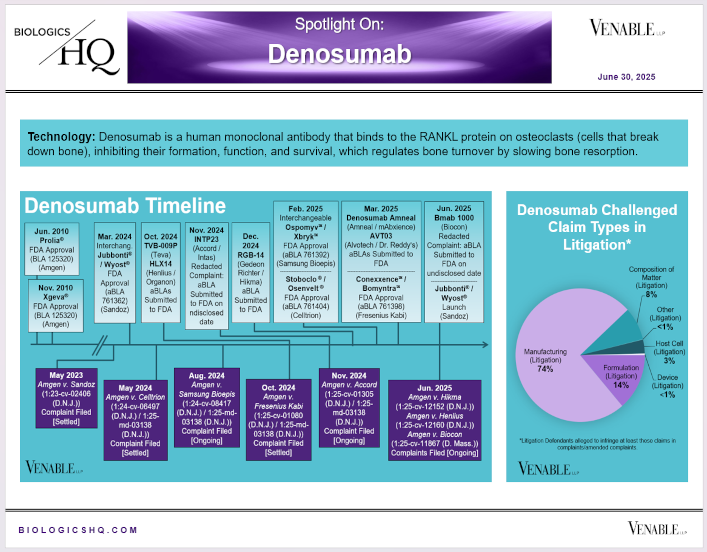
by April Breyer Menon | Jun 30, 2025
*Denosumab Challenged Claim Types in Litigation: Claims are counted in each litigation, so claims from the same patent challenged in multiple litigations are counted more than once. Within each litigation a claim is counted only once. Claims in...
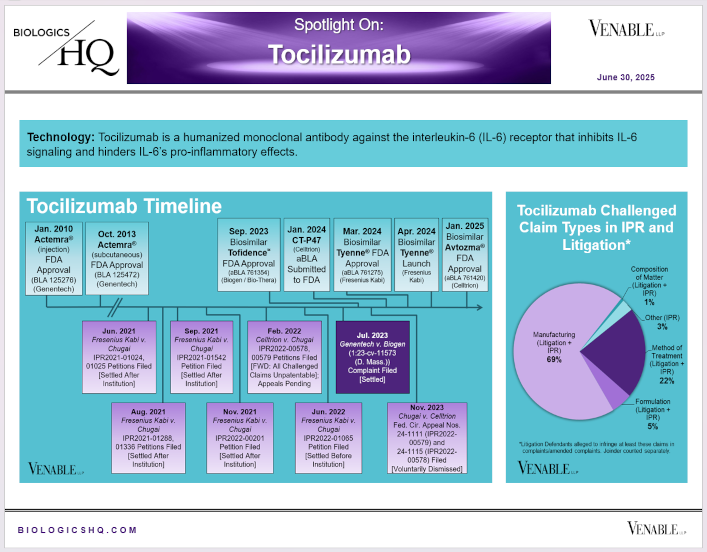
by April Breyer Menon | Jun 30, 2025
*Tocilizumab Challenged Claim Types in IPR and Litigation: Claims include those challenged in litigations and IPRs. Claims are counted in each litigation and IPR, so claims from the same patent challenged in multiple litigations/IPRs are counted more...
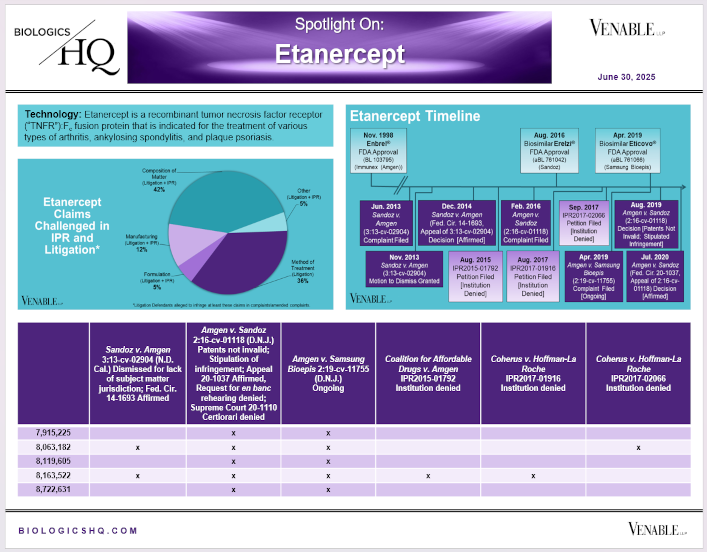
by April Breyer Menon | Jun 30, 2025
*Etanercept Challenged Claim Types in IPR and Litigation: Claims include those challenged in litigations and IPRs. Claims are counted in each litigation and IPR, so claims from the same patent challenged in multiple litigations/IPRs are counted more than...
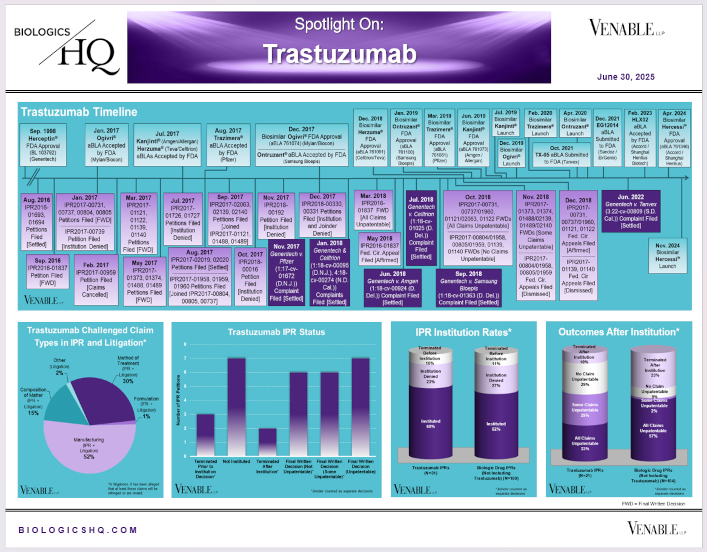
by April Breyer Menon | Jun 30, 2024
*Trastuzumab Challenged Claim Types in IPR and Litigation: Claims include those challenged in litigations and IPRs. Claims are counted in each litigation and IPR, so claims from the same patent challenged in multiple litigations/IPRs are counted...
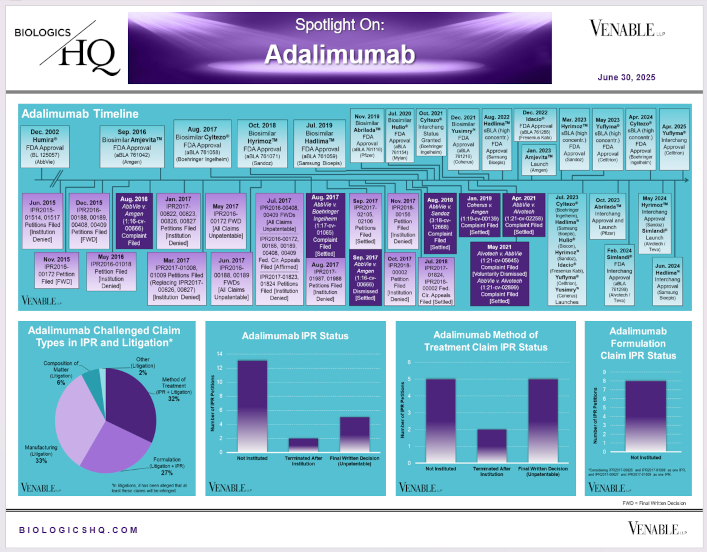
by April Breyer Menon | Jun 30, 2025
*Adalimumab Challenged Claim Types in IPR and Litigation: Claims include those challenged in litigations and IPRs. Claims are counted in each litigation and IPR, so claims from the same patent challenged in multiple litigations/IPRs are...







