The Inflation Reduction Act (IRA), signed into law on August 16, 2022, allows the federal government to negotiate prices for some high-cost drugs covered under Medicare. Under the law, many small molecule and biologic drugs that do not have approved and marketed generics or biosimilars, and that are among the 50 highest-spend Medicare covered drugs for each of Part B and D, can be eligible for negotiated prices nine years (small molecule) or 13 years (biologic) after FDA approval.
On January 27, 2026, the Centers for Medicare & Medicaid Services (CMS) announced the next set of 15 drugs selected for price negotiations for the third cycle of the Medicare Drug Price Negotiation Program. This set of drugs includes drugs covered under Medicare Part D, and for the first time, drugs payable under Medicare Part B. The prices negotiated for these drugs will take effect on January 1, 2028.
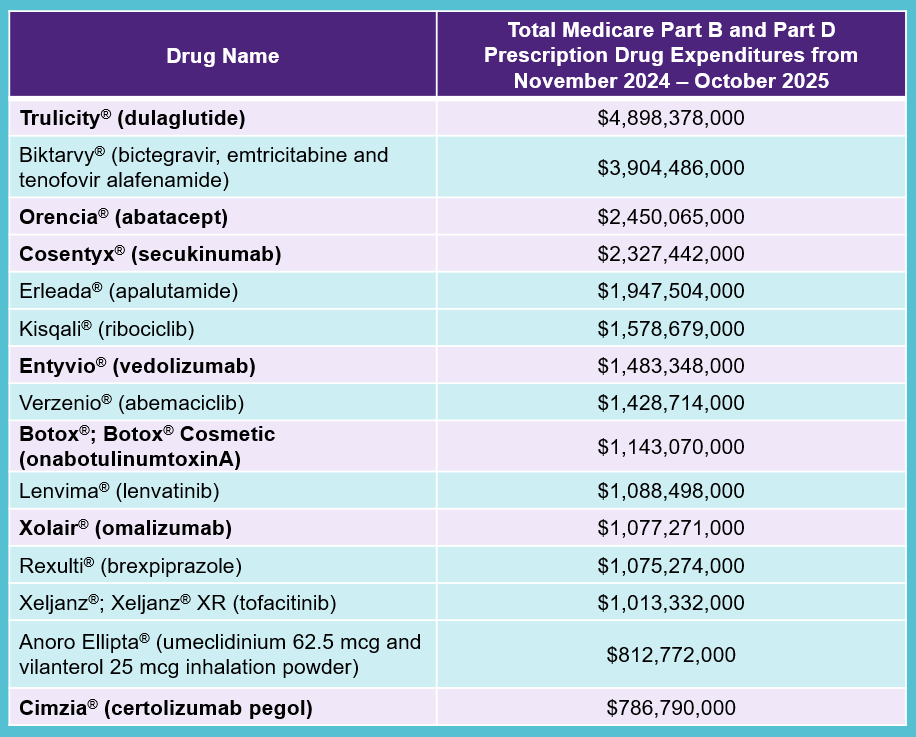
The selected drug list for the third cycle of CMS negotiations, including seven biologics (listed in bold) is:
The selected drug for renegotiation is:
- Tradjenta® (linagliptin)
In addition to releasing the list of the 15 drugs chosen for price negotiations, CMS also released a list of the top 50 negotiation-eligible drugs, which gives insight into which drugs may be chosen in upcoming years.
The first list of drugs for negotiation included 3 biologic drugs, Enbrel® (etanercept), Stelara® (ustekinumab), and Fiasp®; Fiasp® FlexTouch; Fiasp® PenFill; NovoLog®; NovoLog® FlexPen; NovoLog® PenFill (insulin aspart) (previously reported First List of Drugs for Medicare Price Negotiations Published Includes Three Biologics). The second list did not include any biologics. The negotiated prices for the first set of drugs chosen went into effect on January 1, 2026.
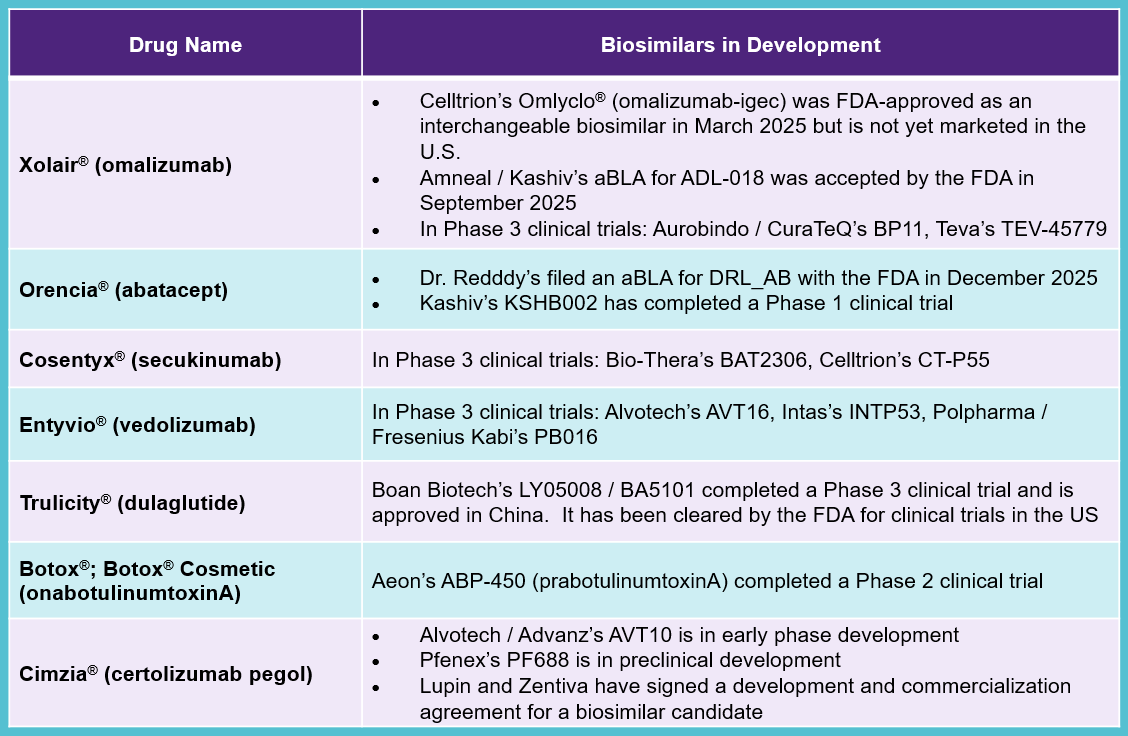
All of the biologics included in the current list have biosimilars in various stages of development. Xolair® already has an approved interchangeable biosimilar along with a pending aBLA and Orencia® has a pending aBLA at the FDA. Therefore, it is possible these drugs will face biosimilar competition prior to negotiated prices taking effect. Currently, there are no pending patent disputes related to proposed biosimilars of the drugs chosen for this round of price negotiations.
Biosimilars in development include:
While prices have been successfully negotiated for drugs on previous lists, at least six drug companies have filed petitions at the Supreme Court related to the constitutionality of the Inflation Reduction Act. To date, drug companies have lost all lower court decisions that have ruled on the merits. Whether the Supreme Court will take any of the cases remains to be seen.
For more information about biosimilars and biosimilar patent disputes, please visit BiologicsHQ.
________________________________________________________________
The author would like to thank April Breyer Menon for her contributions to this article.