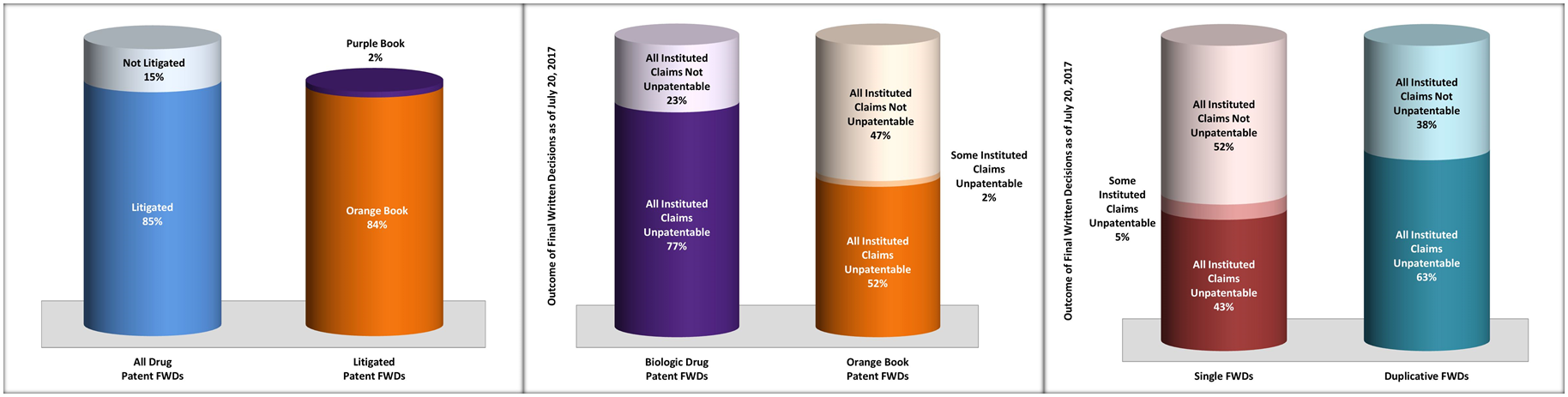
As of July 20, 2017, there have been at least 363 IPR petitions filed against patents that were listed in the FDA Orange Book, and 74 IPR petitions filed against patents that have been identified as reading on FDA Purple Book (CDER) listed biologic drugs. Of these 437 drug patent IPRs, 116 resulted in a final written decision (“FWD”). There are a number of lessons to be learned from these FWDs. We highlight here five of the most interesting:
– Eighty-Five Percent of Drug Patent FWDs Concerned Patents That Have Also Been Litigated
– Patent Claims Have Been Found Unpatentable in More Than Fifty Percent of Drug Patent FWDs
– Sixty-Two Percent of Drug Patent FWDs Have Been Duplicative FWDs With Identical Outcomes
– Drug Patents Challenged in Duplicative FWDs Are More Likely to be Found Unpatentable
– Challenged Claims in Biologic Drug Patents Are More Likely to be Found Unpatentable