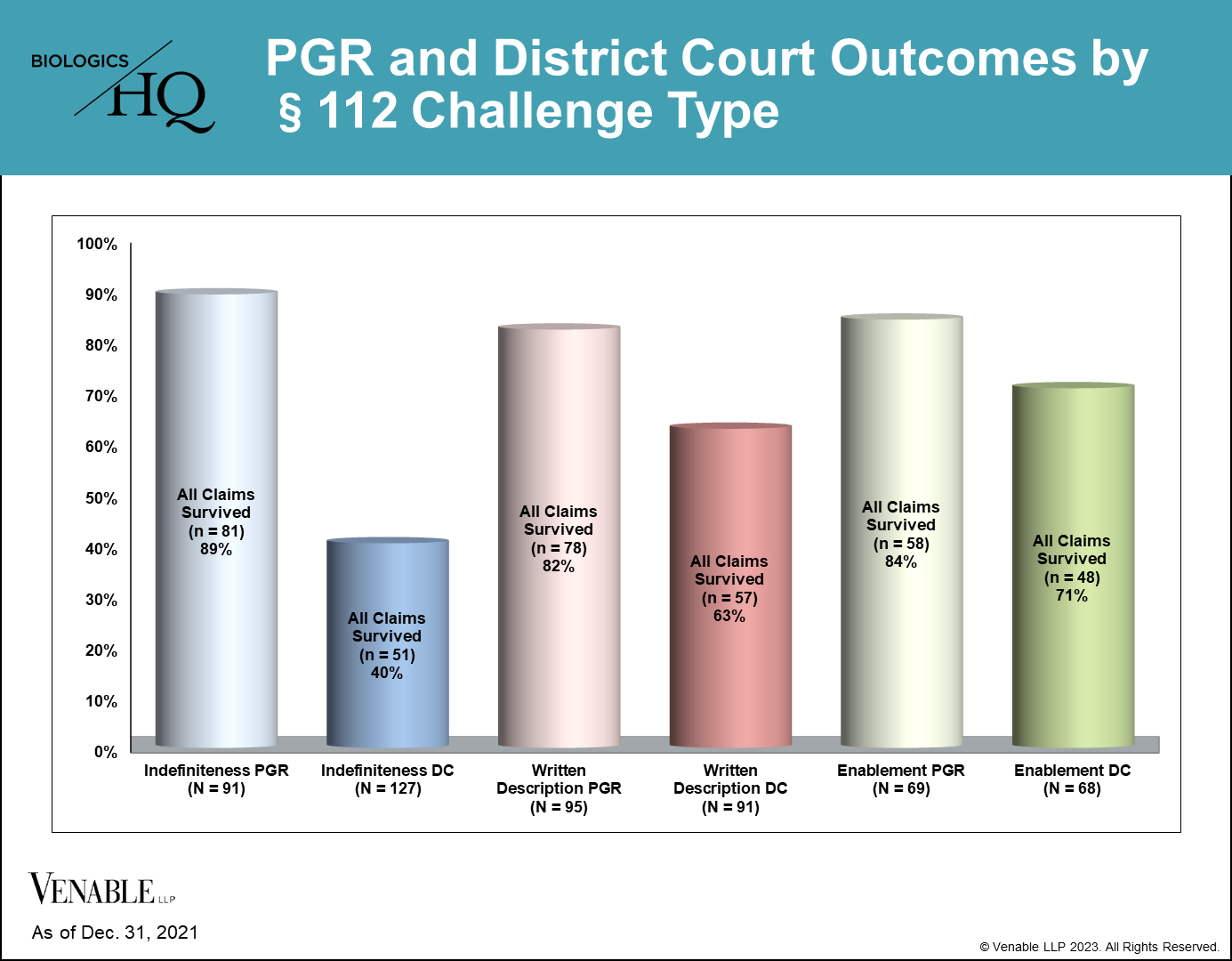
Since § 112 invalidity challenges are available in PGRs we compared PGR § 112 challenge outcomes for indefiniteness, written description and enablement to those challenges in district court (DC) litigations. For this analysis we reviewed district court litigation summary judgment and trial outcomes from January 1, 2015 through December 31, 2021, as this time frame includes essentially all PGR filings. As previously noted, because claims are often challenged under multiple grounds, they are sometimes found not unpatentable under one ground but are invalidated under another ground. Our analysis looks at the outcome of each challenge individually.[1]
As shown in this figure, claims have a higher rate of survival (i.e., no claims unpatentable, considering both DNIs and FWDs) from § 112 challenges at the PTAB compared to in district court. This is most apparent for challenges based on indefiniteness (89% PGR survival, n=81 of 91; 40% DC survival, n=51 of 127) and written description (82% PGR survival, n=78 of 95; 63% DC survival, n=57 of 91). The difference between claim survival in the two venues for challenges based on enablement is slightly less pronounced (84% PGR survival, n=58 of 69; 71% DC survival, n=48 of 68). From the data available as of this writing, which includes 172 PGR outcomes and 134 DC cases with decisions on indefiniteness, written description and enablement challenges, patent challengers have a higher success rate at invalidating claims under § 112 in district court compared to the PTAB. The higher rate of claim survival for a § 112 challenge in district court compared to a PGR is despite the lower burden of proof required to invalidate claims at the PTAB compared to district court (preponderance of the evidence vs. clear and convincing evidence, respectively).
[1] Ns listed for district court litigations are the number of patents with a district court decision (summary judgment or trial) for that particular challenge type.
BiologicsHQ and materials published on BiologicsHQ are published for informational purposes only. Neither the information nor any opinion expressed on BiologicsHQ constitute legal advice, create an attorney-client relationship, or constitute a solicitation for business.

