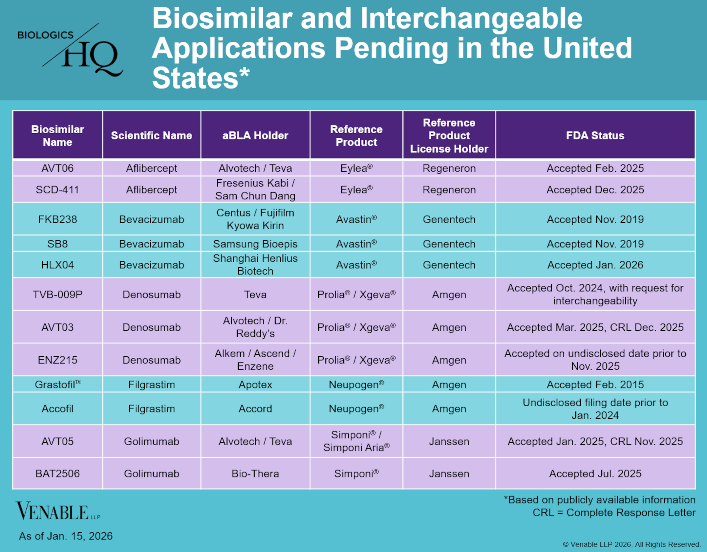
by April Breyer Menon | Jan 23, 2026
BiologicsHQ and materials published on BiologicsHQ are published for informational purposes only. Neither the information nor any opinion expressed on BiologicsHQ constitute legal advice, create an attorney-client relationship, or constitute a...
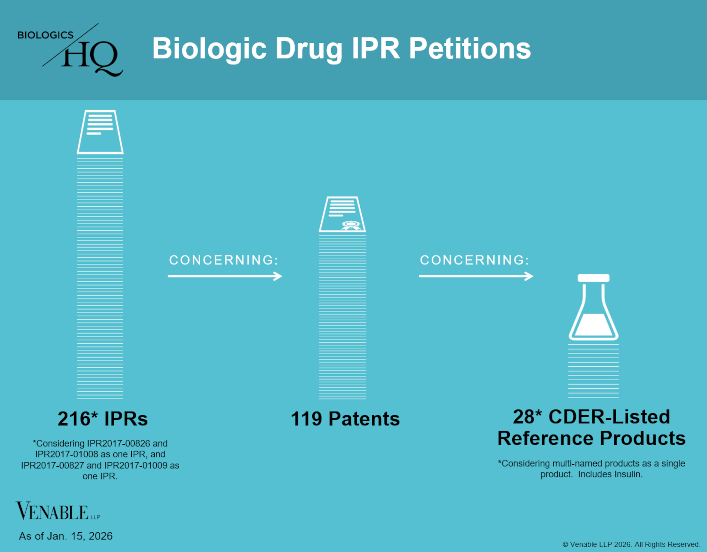
by April Breyer Menon | Jan 23, 2026
Biologic Drug IPR Petitions include IPR petitions relating to CDER-listed biologic products and drugs for which follow-on products are approved under the 505(b)(2) pathway, such as insulin. IPR petitions relating to manufacturing patents that may be relevant...
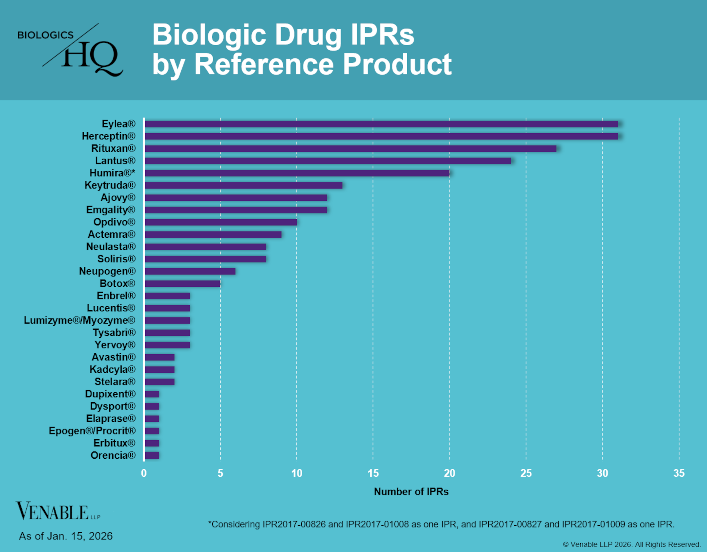
by April Breyer Menon | Jan 23, 2026
Biologic Drug IPR Petitions include IPR petitions relating to CDER-listed / 505(b)(2) biologic products. IPR petitions relating to manufacturing patents that may be relevant to multiple products (for example, U.S. Patent No. 6,331,415 (a “Cabilly” patent)) are...
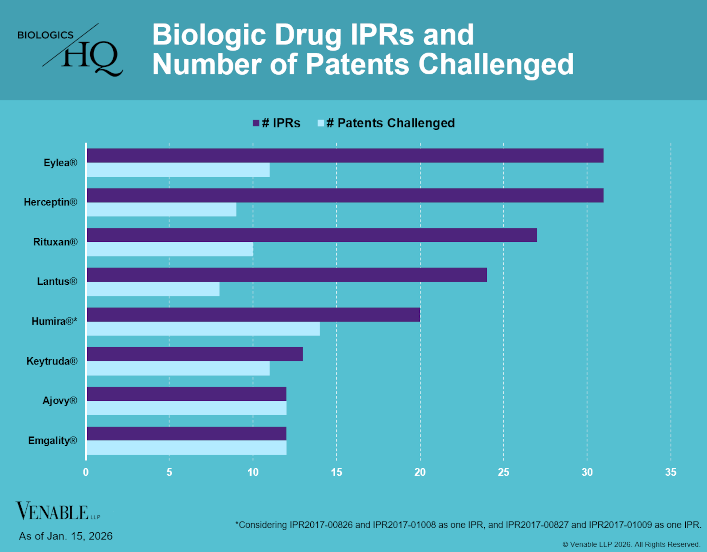
by April Breyer Menon | Jan 23, 2026
Biologic Drug IPR Petitions include IPR petitions relating to CDER-listed biologic products and drugs for which follow-on products are approved under the 505(b)(2) pathway, such as insulin. IPR petitions relating to manufacturing patents that may be relevant...
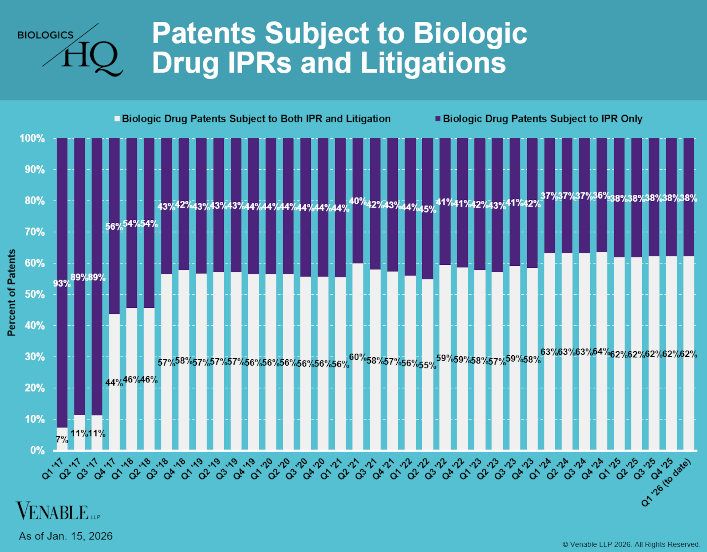
by April Breyer Menon | Jan 23, 2026
Biologic Drug IPR Petitions include IPR petitions relating to CDER-listed biologic products and drugs for which follow-on products are approved under the 505(b)(2) pathway, such as insulin. IPR petitions relating to manufacturing patents that may be relevant to...







