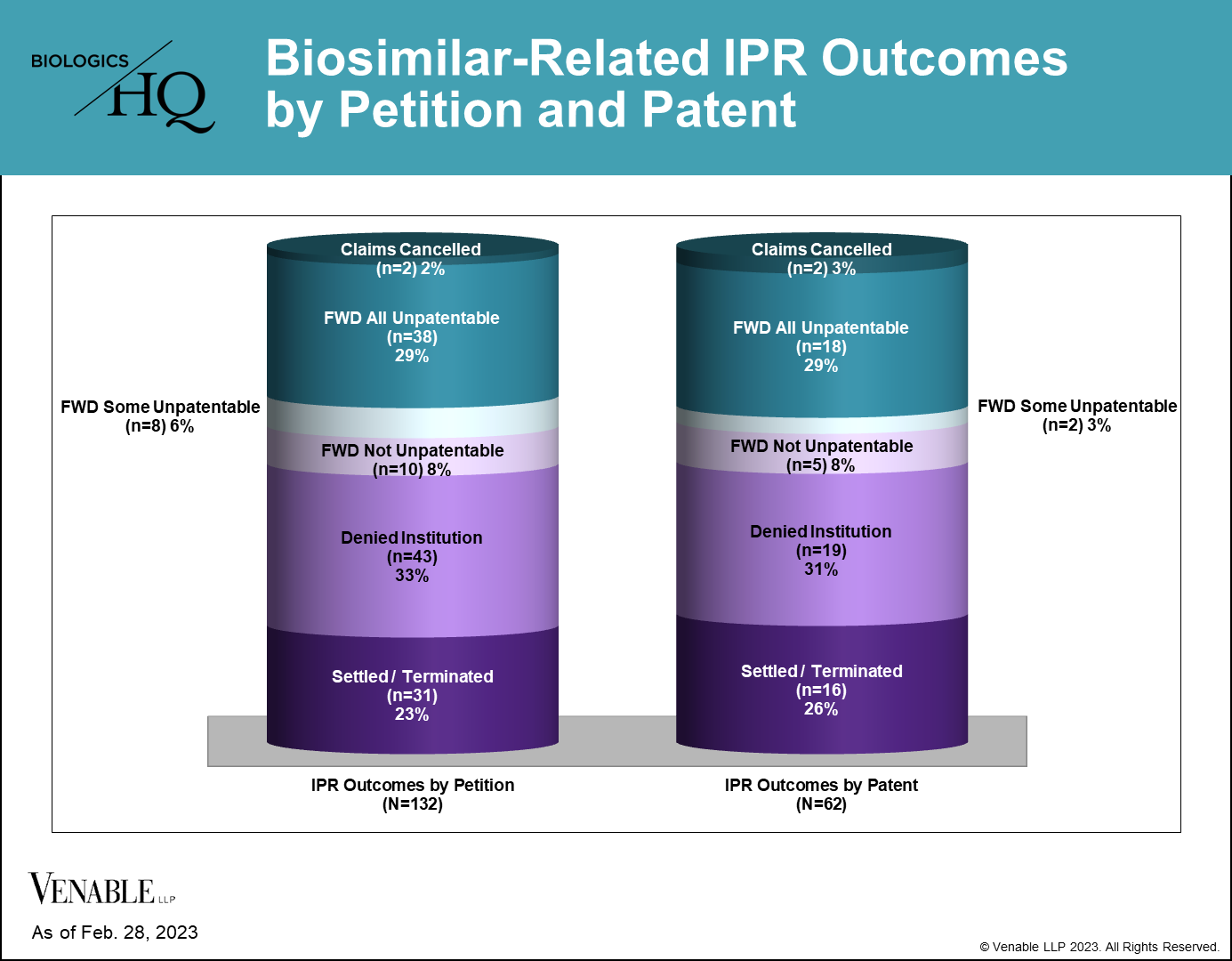
*Biosimilar-related IPR outcomes by petition and by patent. IPRs that are still pending are not included. Settled / Terminated includes IPRs that were settled or terminated at the Petitioner’s request prior to a final written decision, and include IPRs terminated before and after institution decisions. Claims Cancelled refer to IPRs where the Patent Owner requested cancellation of the challenged claims at any point during the IPR. For patents with multiple IPRs having different outcomes, the outcome at the most advanced stage of the IPR is listed (for example, if one IPR on a patent was not instituted but another IPR on the same patent resulted in a FWD, it is listed as a FWD), and the IPR with the most adverse decision to the patent claims is listed (for example, if one FWD found claims not unpatentable and another FWD found the same claims unpatentable, it is listed as unpatentable). Appeal decisions were considered in the outcomes listed.
40 of 62 (64.5%) patents subject to a terminated biosimilar-related IPR survived without cancellation of a single claim. 22 of 62 (35.5%) patents had at least some claims invalidated or disclaimed, related to nine reference products. Of those 22 patents, there were two disclaimers, two patents lost some challenged claims but not all in final written decisions (FWDs), 12 patents were lost in their entirety in FWDs, and six patents lost all challenged claims in FWDs, but maintained the claims that were not challenged in the IPRs.
BiologicsHQ and materials published on BiologicsHQ are published for informational purposes only. Neither the information nor any opinion expressed on BiologicsHQ constitute legal advice, create an attorney-client relationship, or constitute a solicitation for business.