by April Breyer Menon | Nov 6, 2017
Watch Ha Kung Wong, Molly Burich, and Angus Worthing discuss interchangeability as biosimilars enter the rheumatology market in the United States, and remark on perceptions of efficacy and safety of an interchangeable biosimilar versus a biosimilar, as part of the The...
by April Breyer Menon | Nov 3, 2017
Download PDF ...
by April Breyer Menon | Oct 31, 2017
Watch Ha Kung Wong, Angus Worthing, Amanda Forys, and Molly Burich discuss questions regarding physician prescribing of biosimilars and interchangeables, including how the designation of a product as interchangeable will affect prescribing practices and how...
by April Breyer Menon | Oct 30, 2017
Watch Ha Kung Wong, Amanda Forys, and Molly Burich discuss the question, “Will biosimilar manufacturers seeking interchangeability have an effect on how reference product sponsors attempt to protect market share?” as part of the The Center for Biosimilars™...
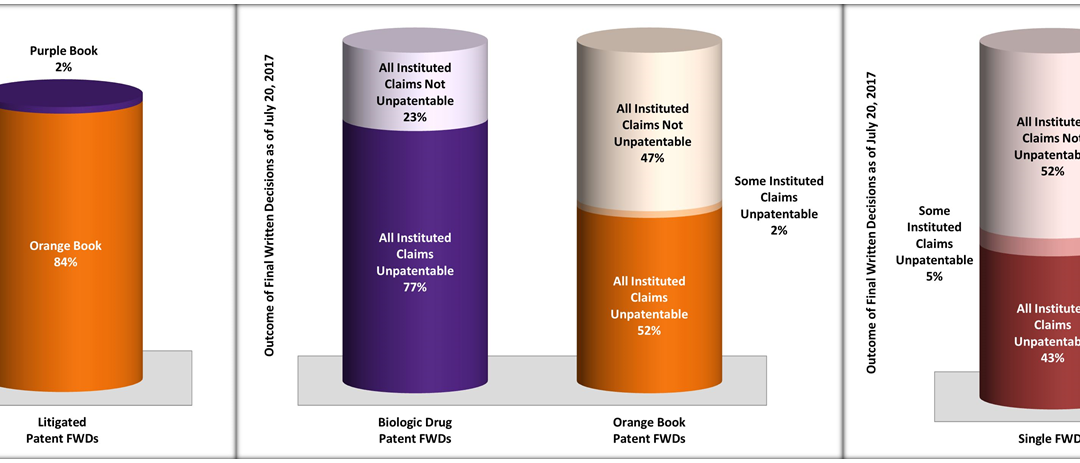
by April Breyer Menon | Oct 23, 2017
As of July 20, 2017, there have been at least 363 IPR petitions filed against patents that were listed in the FDA Orange Book, and 74 IPR petitions filed against patents that have been identified as reading on FDA Purple Book (CDER) listed biologic drugs. Of these 437...
by April Breyer Menon | Oct 5, 2017
Download PDF Download PDF Click on the “Subscribe” link above for future BiologicsHQ Monthly Injections delivered to your inbox.


