by April Breyer Menon | Oct 30, 2017
Watch Ha Kung Wong, Amanda Forys, and Molly Burich discuss the question, “Will biosimilar manufacturers seeking interchangeability have an effect on how reference product sponsors attempt to protect market share?” as part of the The Center for Biosimilars™...
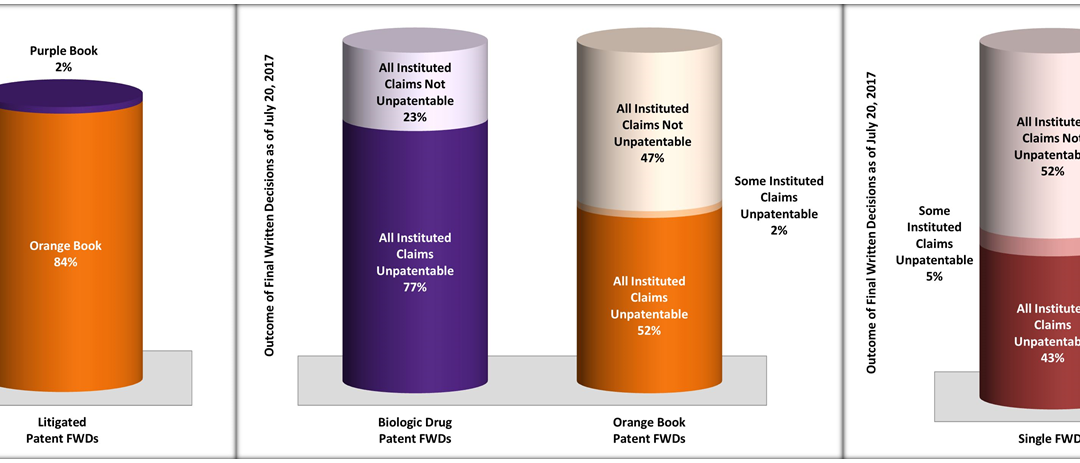
by April Breyer Menon | Oct 23, 2017
As of July 20, 2017, there have been at least 363 IPR petitions filed against patents that were listed in the FDA Orange Book, and 74 IPR petitions filed against patents that have been identified as reading on FDA Purple Book (CDER) listed biologic drugs. Of these 437...
by April Breyer Menon | Oct 5, 2017
Download PDF Download PDF Click on the “Subscribe” link above for future BiologicsHQ Monthly Injections delivered to your inbox.

by April Breyer Menon | Sep 27, 2017
On September 22, 2017, a District of Delaware jury in the matter Amgen v. Hospira, 15-cv-839-RGA (D. Del.) returned a verdict awarding Amgen $70 million for Hospira’s infringement of an Amgen patent covering the manufacture of Amgen’s erythropoetin product Epogen®....

by April Breyer Menon | Sep 21, 2017
On September 21, 2017, a Federal Circuit panel rejected an Eastern District of Texas judge’s proposed four-factor test for determining whether venue is proper over a defendant in a patent infringement action under the “regular and established place of business” prong...




