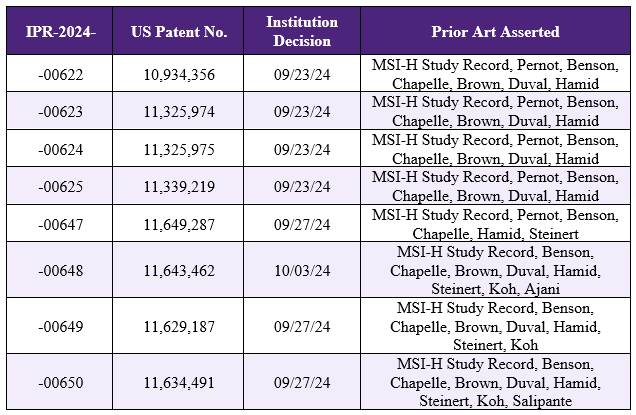
Earlier this year, Merck Sharp & Dohme, LLC (“Merck”) requested inter partes review (“IPR”) of a number of patents owned by the Johns Hopkins University (“JHU”). The patents are directed to methods of treating cancers in patients with high mutational burdens, such as microsatellite instable (“MSI”) cancer, by using anti-PD-1 antibodies, which includes pembrolizumab that is sold by Merck under the trade name Keytruda®. Previously, we reported that in June 2024 the PTAB granted institution of one such IPR – IPR2024-00240 – concerning JHU’s U.S. Patent No. 11,591,393 (see PTAB Grants Institution of IPR Challenging The Johns Hopkins University Pembrolizumab Patent). The PTAB has now granted institution of all of the remaining IPRs filed by Merck based on similar grounds as the -00240 IPR, summarized in the Table below. Institution was granted for both anticipation (35 U.S.C. § 102) and obviousness (35 U.S.C. § 103) challenges in each IPR.
Petitioner used the prior art “MSI-H Study Record” as evidence of anticipation and obviousness in each IPR. This prior art is the study record of a clinical trial sponsored by Merck and JHU using Keytruda® in MSI positive cancer patients, including those with MSI positive colon cancer. The PTAB found that because the treatment of the patients was performed only after microsatellite instability-high (“MSI-H”) status was determined, there is evidence that the MSI-H Study Record teaches treating the patients “in response to” determining their MSI-H status.
Additionally, the PTAB accepted Petitioner’s challenges of the JHU patents based on various combinations of prior art references where Merck alleged that the references taught:
- that PD-1 inhibitors are inherently more effective when treating tumors comprised of cells that are easy for immune cells to recognize (the Brown reference)
- that MSI-H cancers have cells that are easy for immune cells to recognize (the Duval reference)
- clinical practice guidelines for patients with colorectal cancer, including the processes of conducting clinical studies, and that colorectal cancer patients with metastatic and advanced disease who had progressed after previous therapies would be the patient population who would take part in clinical studies (the Benson reference)
- testing techniques to determine MSI (the Chapelle reference)
- intravenous infusion of pembrolizumab (the Hamid reference)
- that patients with MSI-H tumors would be good candidates for immunotherapy (the Pernot reference)
- testing body fluid to determine whether a tumor is MSI-H (the Steinart reference)
- clinical practice guidelines for patients with endometrial cancer and the types of patients who would be enrolled in a clinical trial (the Koh reference)
- testing for HER2 status and selecting systemic therapy based on patient’s performance and HER2 status (the Ajani reference)
- testing tumors for MSI-H status using PCR or next generation sequencing (the Salipante reference)
The co-pending Maryland litigation, Case No. 1:22-cv-03059 (D. Md.), where Merck requested declaratory judgment of noninfringement of all the patents in the instituted IPRs (among other claims), was stayed in its entirety in June 2024 pending resolution of IPR2024-00240.
Keytruda® was the world’s top selling drug in 2023, with over $25B USD in world-wide sales.
We continue to monitor these IPRs and will provide updates when available. For more information concerning these and other biologic drug patent disputes, please visit BiologicsHQ.
_____________________________________________________
The author would like to thank April Breyer Menon for her contributions to this article.