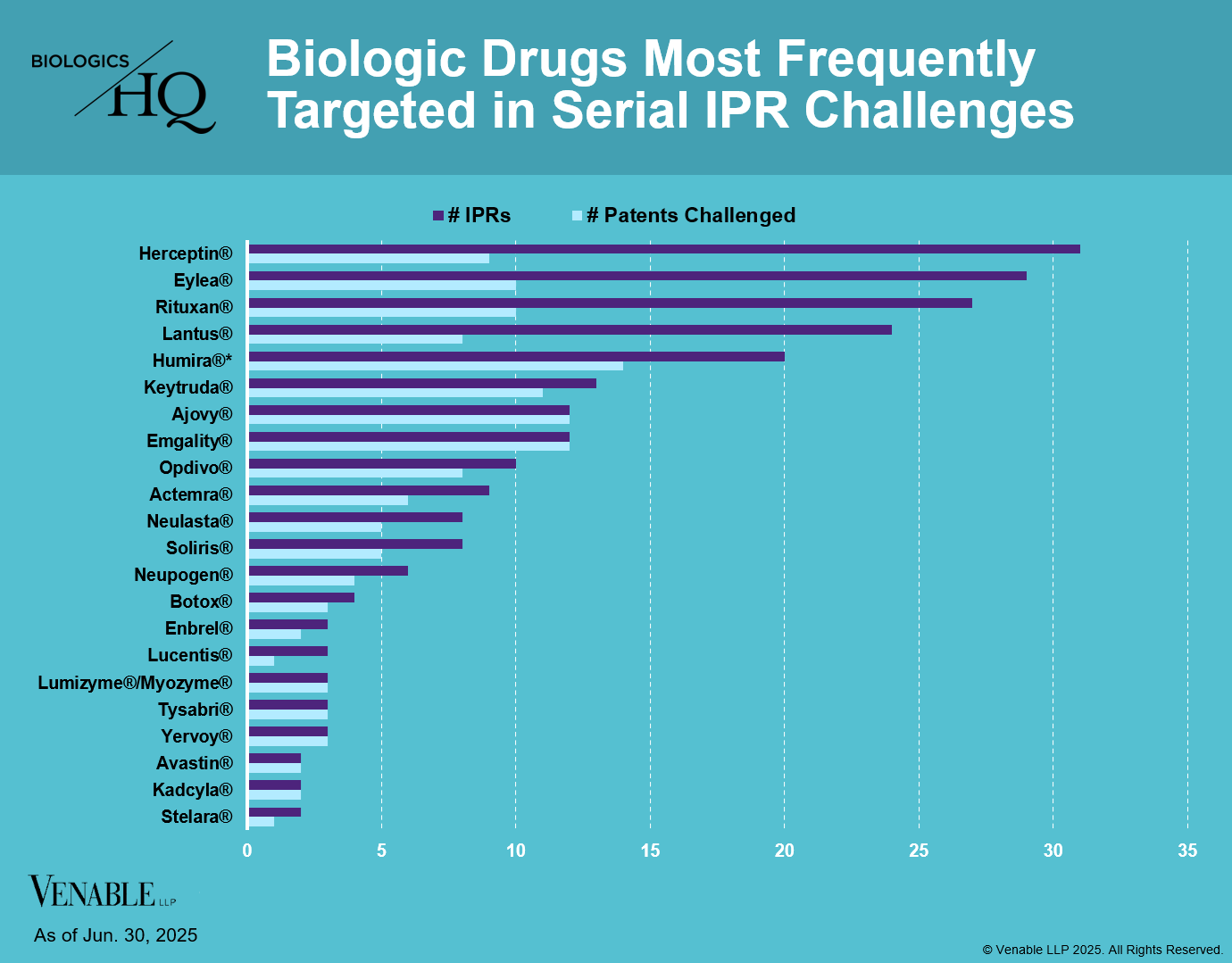
Biologic Drug IPR Petitions include IPR petitions relating to CDER-listed / 505(b)(2) biologic products. IPR petitions relating to manufacturing patents that may be relevant to multiple products (for example, U.S. Patent No. 6,331,415 (a “Cabilly” patent)) are not included.
BiologicsHQ and materials published on BiologicsHQ are published for informational purposes only. Neither the information nor any opinion expressed on BiologicsHQ constitute legal advice, create an attorney-client relationship, or constitute a solicitation for business.

